The middle of summer seems like a good opportunity to check in on some work from users of the Lucent360™. Previously, we’ve highlighted several Lucent360™ uses including a photocatalytic phosphonylation reaction in batch and flow (Ref 1), acceleration of a cobalt catalysis in the Lucent 360™ (Ref 2) and screening reactions for C-N coupling (Ref 3). And while we will also have more of our own work to share soon, let’s discuss some of the creative uses that our users have found to utilize the Lucent360 for their research. We’ll highlight three papers, each focusing on a different key use for the Lucent360: Reaction method development, Scale up in batch and Photochemistry in flow.
Reaction methodology developments (Temperature and Light intensity control)
Research projects often take place across multiple sites, with work from many researchers or different types and brands of photoreactors. All this data is usually blended as tactfully as possible in the Supporting Info on the path to the ultimate method development. In the early stage of discovery an initial result may be derived from a single lamp shining on a single sample (or a few samples) with unknown light intensity wavelength and temperature or a photoreactor only capable of running one sample at a time. Getting from that first result to an extensive methodology paper generally requires something with a higher throughput and reaction parameter control. With that preamble, we want to discuss this recent work entitled “Mild Strategy for the Preparation of Alkyl Sulfonyl Fluorides from Alkyl Bromides and Alcohols Using Photoredox Catalysis and Flow Chemistry” by Alejandro Gutiérrez-González, Staffan Karlsson, Daniele Leonori, and Mateusz P. Plesniak at AstraZeneca (Ref 4).
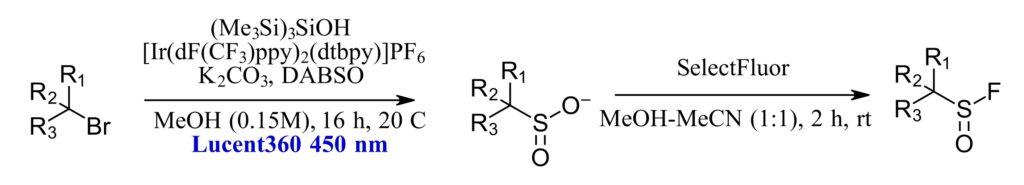
Figure 1: Optimized reaction condition for two-step process to generate sulfonyl fluorides
The method in question is scalable strategy for preparation of alkyl sulfonyl fluorides from alkyl bromides and alcohols (Figure 1). Sulfonyl fluorides are key intermediates as click-like reagents for parallel synthesis or as warheads for covalent inhibitors. The authors set out to create a two-step process to sulfonyl fluorides. The first step involves a photoinduced halogen atom transfer (XAT) with SO2 radical capture, followed by fluorination with SelectFluor. In this scheme, the excited iridium photocatalyst oxidizes the silanol reagent generating a silicon radical reagent that can activate the alkyl bromide. The subsequent carbon radical reacts with the DABSO reagent as the SO2 source resulting in the alkyl sulfinate that can be fluorinated in a second step.
Following initial results with a commercial LED rig, it was observed that while conversion was high (70%) reproducibility between samples was low due to variability in light intensity. This would make method development difficult. We’ll let the author’s own words from the paper establish the importance of the Lucent360™ in this work:
“Switching to a Lucent 360™ photoreactor was crucial in this project because this reactor allows for fine-tuning of the irradiation wavelength, light intensity, and reaction temperature and provides a higher throughput (24 × 4 mL vials).”
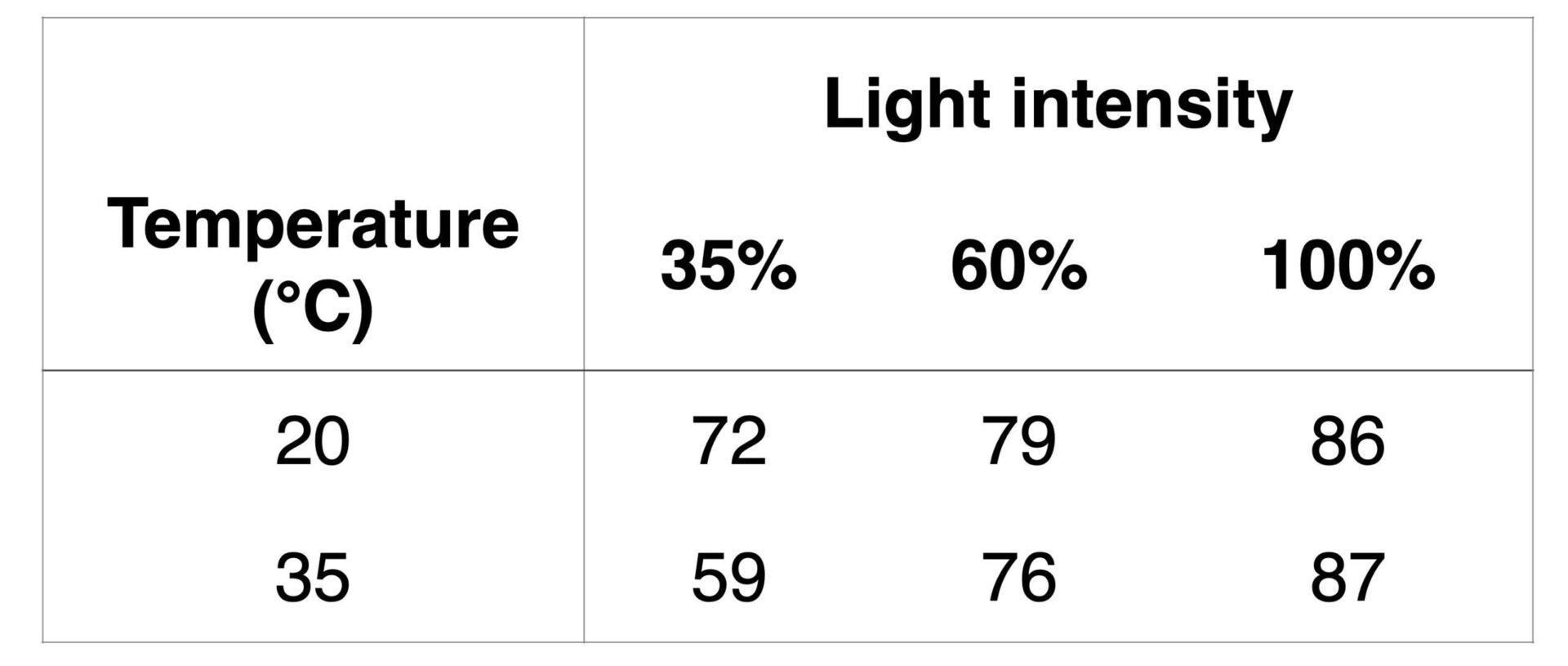
This throughput enabled a screen of catalysts, bases and sulfur radical sources that one would expect, as well as a library of substrates after an optimized condition as determined using the Lucent360™. In the Lucent360™ with the multi-light screening tool, it is possible to screen 4 different light intensities with temperature control in a single experiment 4×4 samples per quadrant. So, it is easy to quickly access data like the following Table below, adapted from the Supporting Info, to demonstrate the connection between light intensity and temperature. The authors screened irradiation intensity and temperature at 35, 60 and 70% at 20 and 35 °C in triplicate with less than 5% variability between trials. Following this a substrate scope included a series of 4-bromopiperidines with N-protecting groups, bromopyrans, bromo-cycloheptane, alkyl bromides and secondary and tertiary bromides. With extensive reaction method development performed in the Lucent360™, the authors were able to take this well-studied reaction and transfer the protocol to a continuous stirred-tank reactor for 5 g reactions of these important sulfonyl fluorides.
Table 1: Comparing temperature and light intensity using the Lucent360™ multi-light screening holder

Scale up in batch (More Light!)
The second work we would like to highlight starts with an EvoluChem Photoredox Box™ for initial reaction discovery and development and transitions to the Lucent360™ for scale up of a key material. The Lucent360™ is part of a suite of EvoluChem™ photoreactors and light sources for running photochemical reactions each with its benefits and limitations and ideal use. The PhotoRedox Box™ is a simple and inexpensive starting point for screening multiple reactions in a single reactor with temperature controlled via a fan at 30 °C. The PhotoRedox Box Duo™ doubles sample capacity, while the PhotoRedox Box TC™ allows temperature control from -10 to 60 °C with an external chiller. The Lucent360™ combines all the best features with light intensity, temperature control and high-through with scale up capabilities in large batch reactions up to a 500 ml reaction.
In their recent work “Deoxy-Arylation of Amides via a Tandem Hydrosilylation/Radical− Radical Coupling Sequence”, Nicholas J. Venditto* and Jeffrey A. Boerth demonstrate reaction screening in EvoluChem PhotoRedox Box™ and scale up capabilities of the Lucent360™ for a two-step deoxy-arylation of amides.
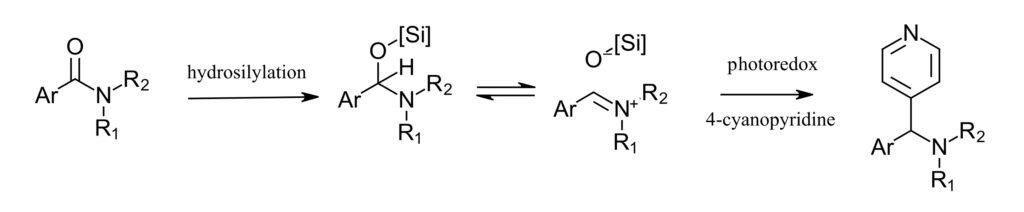
Figure 2: Amide deoxy-arylation. Step 1: hydrosilylation. Step 2: radical couple with photoredox
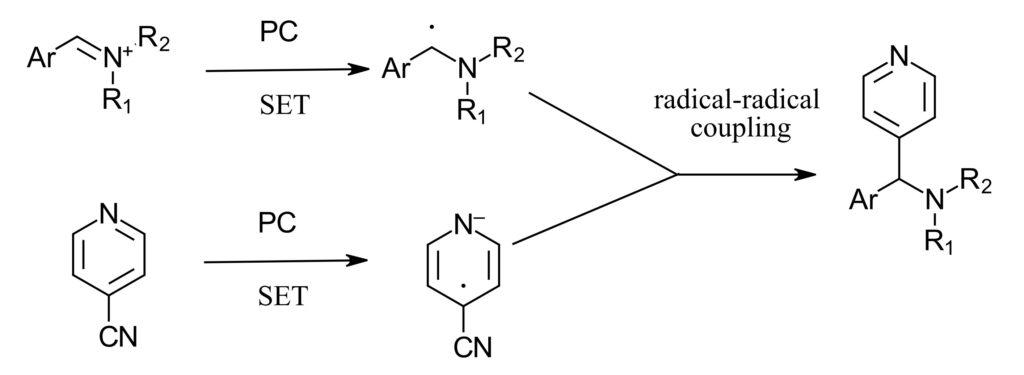
The reaction scheme proceeds in two steps, first hydrosilylation of amide. Here the authors use Vaska’s complex (IrCl(CO)(PPh3)2) and TMDS. For the photoredox step, both the 4-cyanopyridine and the α-amino radical can form in the same photoredox cycle. With this scheme in hand, the authors investigated the photoredox portion of the reaction in the PhotoRedox Box™. Here they screened photocatalysts (iridium and organic photocatalysts) reductants (selecting DIPEA) and equivalents of all reagents. With optimized condition in hand, the authors then used a series of amines and arenes to demonstrate substrate scope and late-stage functionalization of a few biologically relevant compounds such as DEET and paroxetine.
Figure 3: Photoredox scheme
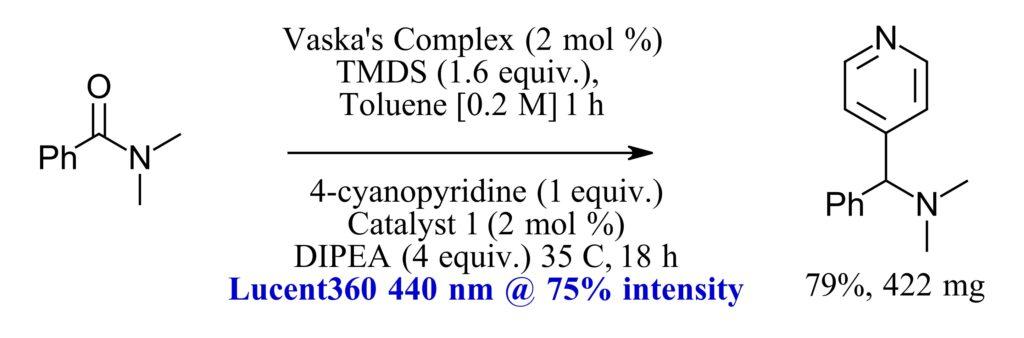
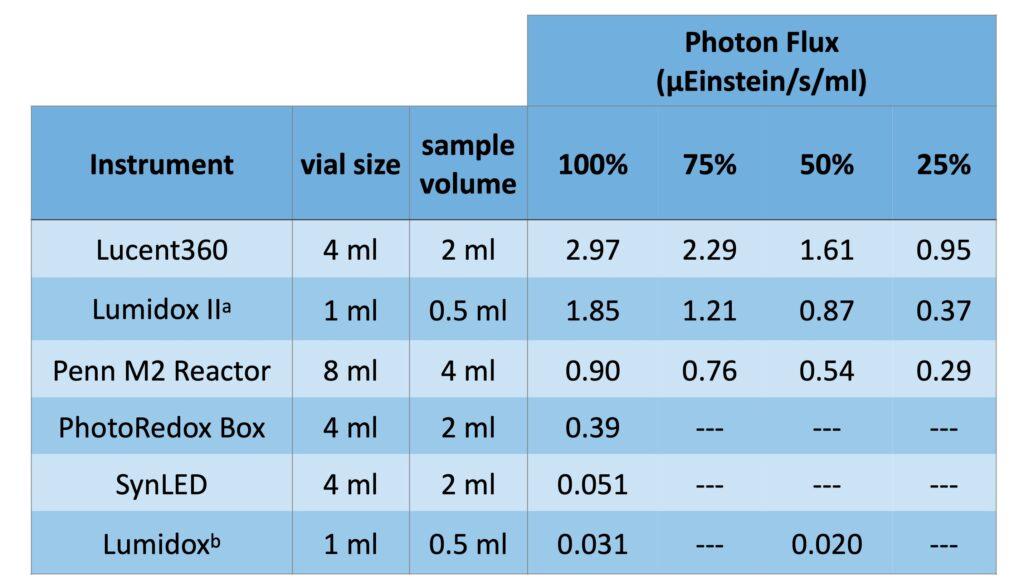
For scale up of this method, the authors found that higher light intensity was needed due to the darkening of the reaction mixture. This is where the Lucent360 comes into play. Transferring the reaction to the Lucent360™ the authors were able to prepare the material in Figure 4 at 79% yield (422 mg) due to the higher light intensity found in the Lucent360™. While not found in the paper cited, we do have some insight we can share on this observation. By using ferrioxalate actinometry, we’ve compared all the commercial photoreactors that we can find and the Lucent360™ has highest photon flux that we’ve been able to measure (Table 2). For a reaction that requires more light, the Lucent360 can be the best option.
Figure 4: Amide functionalization via hydrosilylation. Scale up in Lucent360™
Table 2: Ferrioxalate Actinometry data comparing commercial photoreactors (450 nm) as measured at Hepatochem™.
a. Settings 650 mW, 460 mW, 350 mW, 120 mW.
b. Settings at 30 mA and 20 mA
Polymer Photochemistry in Flow (Higher Throughput!)
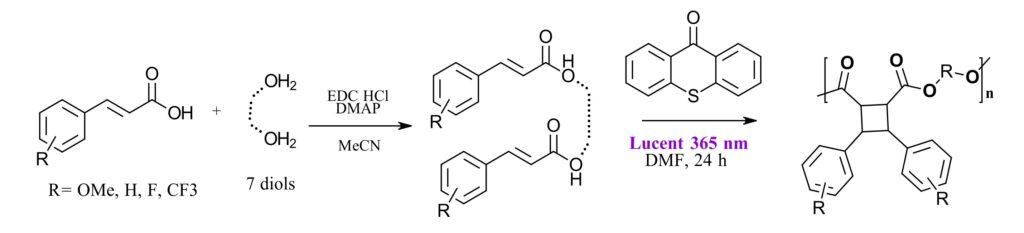
The third example comes from outside our world of small molecule synthesis and looks at polymer photochemical synthesis in batch with transition to flow. In their work entitled, “Accessing Cyclobutane Polymers: Overcoming Synthetic Challenges via Efficient Continuous Flow [2+2] Photopolymerization” Aaron B. Beeler, Mark W. Grinstaff and coworkers at Boston University use the Lucent360™ equipped with a flow cell to produce a library of complex truxinate cyclobutene polymers including 36 that have previously not been synthesized before. They were able to take advantage of a key feature of the Lucent360™, interchangeable sample holders for reaction volumes from 2 ml up to 500 ml for batch reactions with the option for running flow chemistry in the same instrument.
Figure 5: Intermolecular [2+2] photocycloaddtion
These cyclobutene-based polymers are of interest due to their potential as biodegradable sustainable polymers. There’s a whole lot of detail in the paper regarding how these polymers have been made previously, including solid state methods, photochemistry and solution state methods, and limitations for the types of monomers that are suitable for reactivity. But the key take away is that the authors were looking for a solution based photochemical method suitable to a diverse set of starting materials and linkers giving controlled molecular weights and dispersity control for diverse products.
The authors investigated cinnamic acid monomers, various linkers, reaction molarity, photosensitizer in batch and flow. Using the Lucent360™ with 365 nm LEDs and temperature control between 12-18 °C, irradiation time was investigated from 6-36 hours. 24 hours was chosen as the optimal reaction time giving polymers with greater than 30kDA weight and dispersity less than 3. For 6 cinnamic acids and 7 diols in batch, issues were observed with reactivity across the series as well as some undesirable polymer properties. And so, the reaction was transferred to a continuous flow cell inside the Lucent360™.
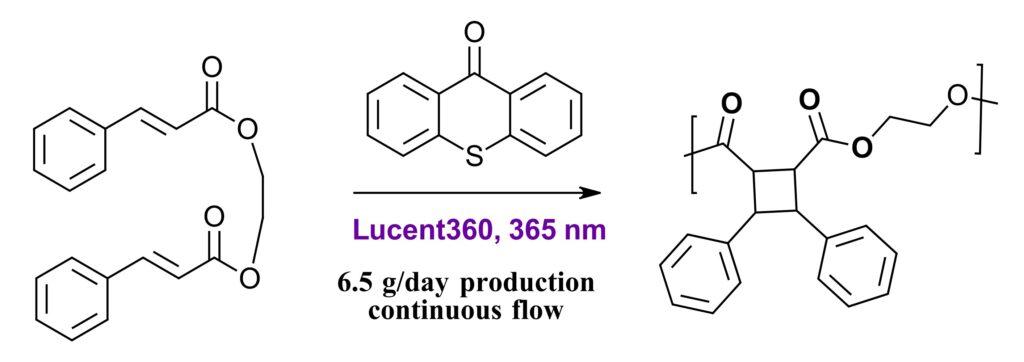
By adjusting flow rates, residence times of 6, 12, 18, 24 and 36 hours were investigated for the polymerization inside the Lucent360™ flow cell. With increased residence time, a logarithmic increase in molecular weight occurs, however molecular weights were significantly larger than in flow (7.9-181.3 kDa) to batch (5.5-64.3 kDa) with smaller dispersities in flow than batch. The benefits of continuous flow to batch are further demonstrated by the synthesis of a polymer based on cinnamic acid and 1,2 ethanediol. Here 6.5 g/day of polymer was produced in continuous flow in the Lucent360™ compared to 100-200 mg per day in batch (Figure 6).
Figure 6: Continuous flow synthesis of cyclobutene polymer in Lucent360™
With the library of polymers in hand, the authors proceed to report all kinds of analysis and parameters for evaluated the polymers which is well outside the scope of what we understand here. The general take-away? The polymers synthesized in flow in the Lucent360™ demonstrated better properties than those in batch, with polymers that couldn’t be synthesized via any other methods now available for investigation for potential uses.
The Lucent360™ is the first reactor to enable batch chemistry and flow chemistry in the same device. Interchangeable LED modules allow experiments to be performed from 254 nm to 740 nm giving flexibility to the user with experiment design. Using the multi-light screening tool, the user can screen multiple wavelengths and light intensities with a single experiment, quickly generating data for reaction optimization. We can’t predict all the ways that our users will find to take advantage of the flexibility afforded by the Lucent360™ in their own research, but we’re always excited by the results.
References:
-
- Lapierre, R.; Le, T. M. T.; Schiavi, B.; Thevenet, D.; Bazin, M.; Buzdygon, R.; Jubault, P.; Poisson, T. Photocatalytic and Photoinduced Phosphonylation of Aryl Iodides: A Batch and Flow Study. Org. Process Res. Dev. 2023. https://doi.org/10.1021/acs.oprd.2c00379.
- Zhao, H.; Caldora, H. P.; Turner, O.; Douglas, James, J.; Leonori, D. A Desaturative Approach for Aromatic Aldehyde Synthesis via Synergistic Enamine, Photoredox and Cobalt Triple Catalysis. Angew. Chem. Int. Ed. 2022. https://doi.org/10.1002/anie.202201870.
- https://hepatochem.com/choose-your-own-adventure/
- Karlsson, S.; Leonori, D.; Plesniak, M. P. Mild Strategy for the Preparation of Alkyl Sulfonyl Fluorides from Alkyl Bromides and Alcohols Using Photoredox Catalysis and Flow Chemistry. Org. Lett. 2024, 2 (1). https://doi.org/10.1021/acs.orglett.4c01216.
- Venditto, N. J.; Boerth, J. A. Deoxy-Arylation of Amides via a Tandem Hydrosilylation/Radical- Radical Coupling Sequence. Org. Lett. 2024, 26 (17), 3617–3621. https://doi.org/10.1021/acs.orglett.4c01121.
- El-Arid, S.; Lenihan, J.; Jacobsen, A.; Beeler, A.; Grinstaff, M. Accessing Cyclobutane Polymers: Overcoming Synthetic Challenges via Efficient Continuous Flow [2+2] Photopolymerization. ACS MacroLetters 2024. https://doi.org/10.1021/acsmacrolett.4c00083.