Without photoredox chemistry there would be no life on earth. And more importantly, without photoredox chemistry there would be no HepatoChem. So, it feels like a moral requirement that we discuss this fascinating recent work by Oliver Trapp and coworkers at the Ludwig-Maximilians University in Munich. Their Accounts of Chemical Research article “(Photoredox) Organocatalysis in the Emergence of Life: Discovery, Applications, and Molecular Evolution” describes the role of photoredox organocatalysis in prebiotic chemistry (Ref 1). And yes, the title of our blog post is highly reductive. But if you clicked, it got your attention. There’s so much more here than shining an LED on things.
How did life on earth begin?
Few questions are more fundamental than the origin of life. Scientists in many fields have their own unique approach to studying this question. Astronomers can use giant telescopes to look deep into the early universe to see how planets form for insight on early conditions on earth. Archaeologists can dig holes in the ground to find fossilized microorganisms and date them back billions of years. Geneticists can sequence the entirety of the genetic code and decipher the implications for where we all started. Chemists can do what we always do, study chemical reactions, and make things.
Oliver Trapp and coworkers are interested in questions related to chemistry on a prebiotic earth and the extremes of the universe. Asking questions like what happens in the chemistry of polycyclic hydrocarbons in exoplanet’s atmospheres (Ref 2), how CO2 fixation could exist on iron-rich meteorites, (Ref 3) or developing a device capable of mimicking the electronic discharges that occur during volcanic lightning strikes to study prebiotic synthesis (Ref 4). In their recent Account, they weave together the story of their recent work to tell the story of how early prebiotic chemistry enabled the formation of photoredox organocatalysts that could evolve, catalyze the formation of new building blocks and catalyze key reactions for generating molecular complexity and potentially life.
The question of where life began on a molecular level can be approached from two directions, from top down or from bottom up. “Top down” looks at the complexity of existing organisms and reduces it to its simplest viable living “protocell”. We’ll quote the authors here to describe a simple system considered to be alive:
“A system’s functionalities that are considered characteristic for life are evolution, self-reproduction, metabolic activity, and compartmentalization. The challenges that therefore need to be met by a system to be considered prebiotically relevant are the formation of random sequences and the ability to copy and to replicate.” (Ref 1)
Trapp and coworkers are working from a bottom-up approach, starting with a few select molecules that are known to exist in a prebiotic environment and working up from there to get increasingly complex molecules ultimately arriving at the “protocell”. For this work, they set out with the goal to find a plausible prebiotic organocatalyst and study its role in molecular evolution. The photoredox component may have been a happy accident.
The search a prebiotic photoredox organocatalyst
As the hypothetical prebiotic chemist on Day 0 charged with designing a chemical synthesis to create new material, what do you have to work with? To start, the Trapp group recently described the formation of simple formaldehyde and acetaldehyde compounds and higher organics from fixation of CO2 catalyzed by iron particles derived from either meteorites or volcanic activity (Ref 3) (Figure 1A). This experiment is fascinating in and of itself using ground up iron meteorites and volcanic ash from Mt. Etna in a custom high-pressure system designed to mimic possible atmospheric conditions on early earth. We must admit, reading about a reaction screen with catalysts derived from different meteorites of different chemical composition is first for us. From the resulting aldehydes and ketones, with the presence of the readily available hydrogen cyanide and aqueous ammonia a pool of α-aminonitriles can be formed (Figure 1B). And finally, a reaction with a second carbonyl in presence of readily available hydrogen sulfide and ammonia can give imidazoline-4-thione compounds (Figure 1C) (Ref 5).
Figure 1: A. Formation of formaldehyde and acetaldehyde from iron meteorites or volcanic ash (Ref 3). B. Creating a pool of α-aminonitriles, C. Formation of imidazolidine-4-thione derivatives.
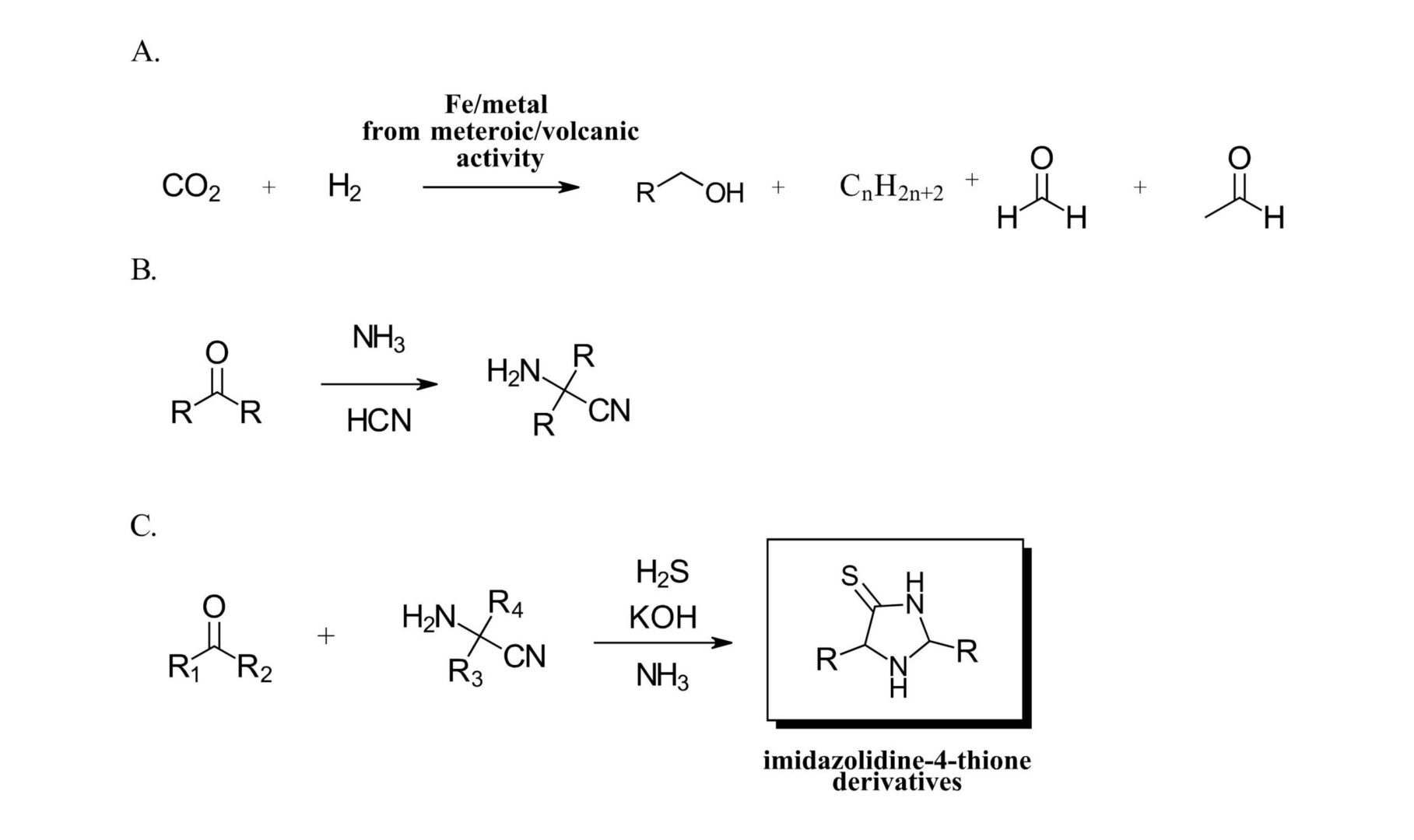
With this reaction scheme, the authors set out to synthesize a library of 16 imidazolidine-thione compounds from aldehydes and ketones with readily available small alkyl groups (methyl, ethyl, isopropyl) at the R positions in Figure 1C. During this study, the authors observed several interesting details with implications for further molecular complexity. First, one of the library compounds, 2-ethyl, 4-methyl-imidazolidine-4-thione afforded enantiomerically pure crystals from a racemic mixture, an interesting phenomenon that could lead to the emergence of chirality in an achiral system. Second, scrambling was observed between the α-aminonitriles and aldehydes further increasing product complexity. In addition, a significant amount of work was performed looking at mechanistic implications, reactions on pools of mixtures and further study of the products.
Prebiotic Photoredox Catalysis
With their library of catalysts on hand, the authors looked to the work of Nobel prize winning organocatalysis and photoredox experts from the David MacMillian group for the α-alkylation of aldehydes (Ref 6). This reaction was chosen in part due to the intriguing potential of the catalyst to modify its own potential building blocks, which could present the possibility for molecular evolution. The original reaction in the MacMillian work called for a ruthenium catalyst, lutidine and degassed solvents. After some reaction development to modify the MacMillian reaction to remove their photocatalyst and solely use the imidazolidine-thione catalysts with prebiotically available reagents in air, the authors screened their catalyst library on propanal and 2-bromoacetonitrile with a 365 nm LED (The LED in the title, from the beginning of the blog post!) to simulate the UV-A radiation of the sun. (Figure 2) If the presence of 2-bromoacetonitrile looks funny to you, don’t fret, the authors have you covered. This is apparently formed as a known reaction of interstellar bromine and acetonitrile and is fair game for this work. Product was observed for all catalysts screened with conversions as high as 78% and 65% ee. The resulting nitrile compounds can be hydrolyzed and/or the aldehydes further oxidized diversifying the product pool and generate new catalysts (Figure 3).
Figure 2: Imidazolidine-thione photoredox organocatalytic alkylation of aldehydes.
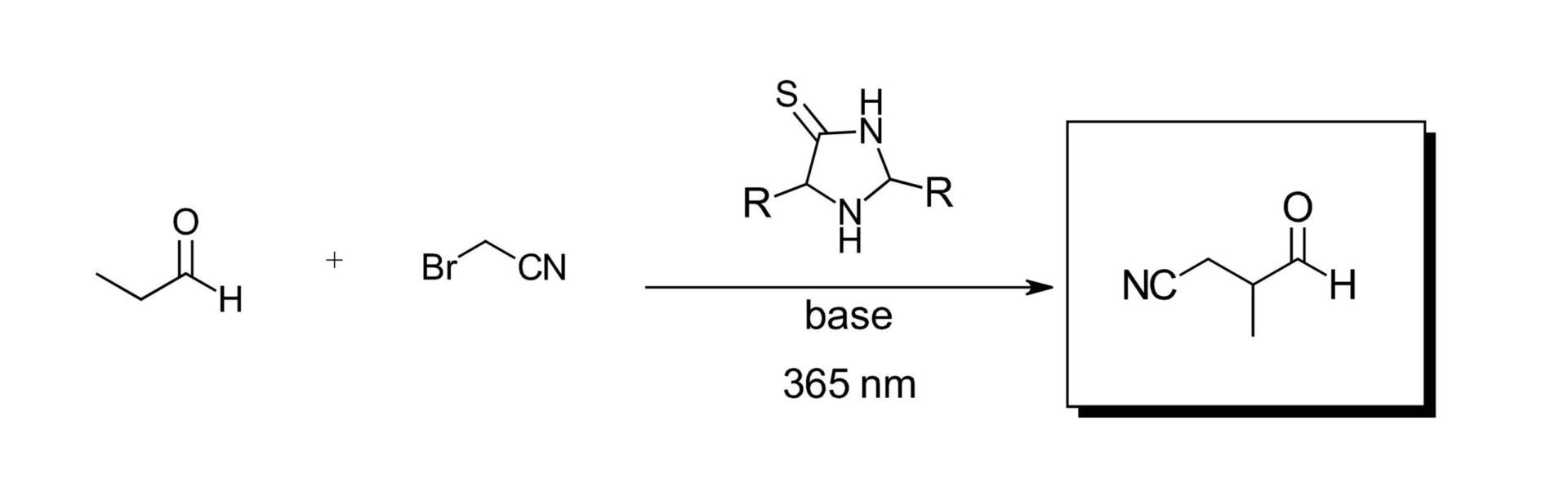
Figure 3: Proposal for generating new building blocks and catalysts using photoredox organocatalysis
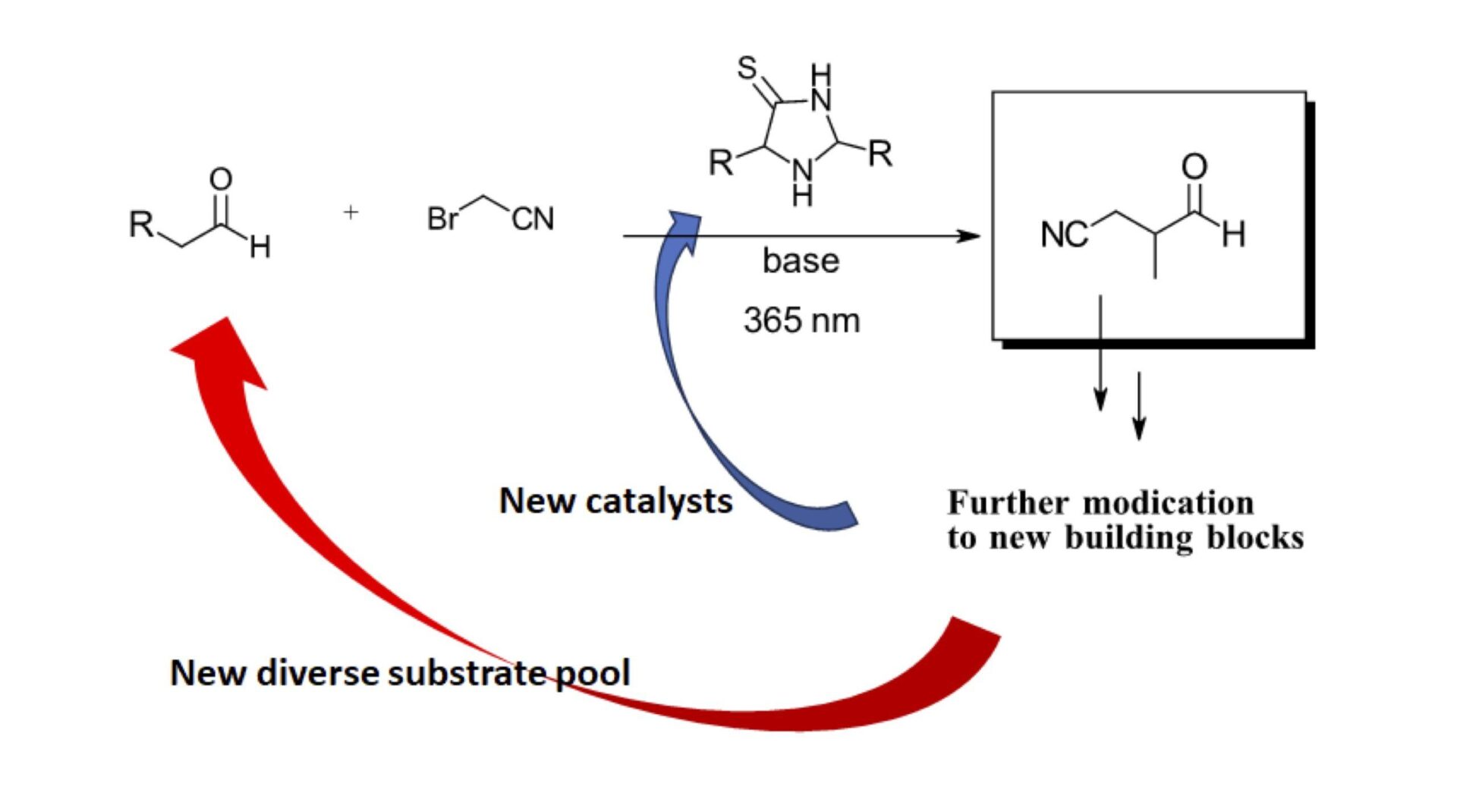
Want to see increasingly complex thione 2nd and 3rd generation catalysts from feasibility derived feedstocks? Well, it’s here as well but we’ll spare you the details as we can’t do the full work justice in this short summary. There’s also an extensive study of the active photosensitizer species in each of these reactions and implications on inducing further molecular complexity. Want to see these increasingly complex thione catalysts catalyze the phosphorylation of nucleosides, a significantly relevant precursor to larger biomolecules, it’s all here in extreme detail, under a wide range of possible prebiotic conditions.
These are just a few examples in the Trapp group’s account that looks to place organocatalysis, with the broad range of diverse and highly selective reactions possible from a prebiotic pool of materials, as a key step between prebiotic chemistry and enzymatic catalysis. Their reactions are a key step towards the formation of complex amino acids, chiral materials and catalyst evolution leading towards life. The photochemical aspect of the organocatalysis with reactions both working in light and dark, demonstrates reaction diversity that can occur during day/night cycles and extreme environments on early earth. All in all, just an incredibly interesting telling of the story of the group’s work.
Inspired? Check out our new selection of LEDs!
Send us your thoughts at info@hepatochem.com
References:
(1) Bechtel, M.; Ebeling, M.; Huber, L.; Trapp, O. (Photoredox) Organocatalysis in the Emergence of Life: Discovery, Applications, and Molecular Evolution. Acc. Chem. Res. 2023. 56, (20) 2801-2813. https://doi.org/10.1021/acs.accounts.3c00396.
(2) Dubey, D.; Grübel, F.; Arenales-Lope, R.; Molaverdikhani, K.; Ercolano, B.; Rab, C.; Trapp, O. Polycyclic Aromatic Hydrocarbons in Exoplanet Atmospheres. I. Thermochemical Equilibrium Models. Astron. Astrophys. 2023, 53, 1–10. https://doi.org/10.1051/0004-6361/202346958.
(3) Peters, S.; Semenov, D. A.; Hochleitner, R.; Trapp, O. Synthesis of Prebiotic Organics from CO2 by Catalysis with Meteoritic and Volcanic Particles. Sci. Rep. 2023, 13 (1), 1–15. https://doi.org/10.1038/s41598-023-33741-8.
(4) Springsklee, C., Scheu, B., Seifert, C., Cimarelli, C., Gaudin, D., Dingwell, D. B., and Trapp, O.: Experimental volcanic lightning under conditions relevant to the early Earth: Discharges as a possible prebiotic synthesis mechanism, EGU General Assembly 2023, Vienna, Austria, 24–28 Apr 2023, EGU23-1742, https://doi.org/10.5194/egusphere-egu23-1742, 2023.
(5) Closs, A. C.; Fuks, E.; Bechtel, M.; Trapp, O. Prebiotically Plausible Organocatalysts Enabling a Selective Photoredox α-Alkylation of Aldehydes on the Early Earth. Chem. – A Eur. J. 2020, 26 (47), 10702–10706. https://doi.org/10.1002/chem.202001514.
(6) Welin, E. R.; Warkentin, A. A.; Conrad, J. C.; MacMillan, D. W. C. Enantioselective α-alkylation of aldehydes by photoredox organo- catalysis: rapid access to pharmacophore fragments from β- cyanoaldehydes. Angew. Chem., Int. Ed. 2015, 54, 9668-9672.

