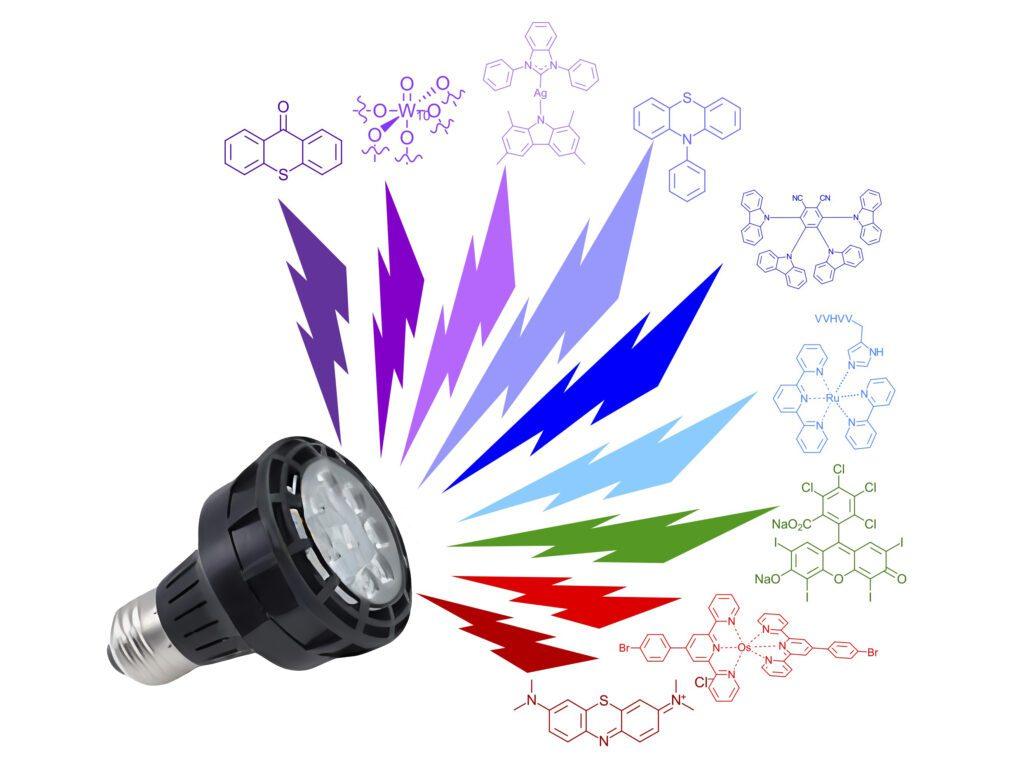
This month, we look at the Photochemistry of the Rainbow — chemical synthesis with purple and red LEDs and everything in between. The photochemistry world is full of color yet can often seems to be dominated by blue LEDs (and iridium catalysts). An expansive world of possible photochemistry exists across the visible spectrum. So, why is everyone always so blue? For the 9th month of the year, we thought we would try out a new angle for the newsletter: 9 recent papers describing 9 reactions using 9 different wavelength EvoluChem™ LEDs with 9 unique catalysts, and not an iridium catalyst to be seen anywhere.
Visible light, depending on whose definition and for what purpose you are describing it, is generally considered to fall somewhere between 380 nm to 780 nm although the sensitivity of the human eye varies across the spectrum. Stare at an EvoluChem™ 365 nm LED without your orange safety glasses and you’ll see a dull purple light. While most of the irradiation is below the sensitivity of your eyes, these bulbs are every bit as bright as the blue LEDs to which you might be accustomed. Likewise, take off your blue safety glasses and the EvoluChem™ 740 nm LED is a dull red color but also still powerful. Of note, the reagents in your flask don’t much care if you can see the photons that are bombarding them. Starting with the 365 nm LEDs as we move to longer wavelengths, the energy of the photons emitted decreases (blame some guy named Planck), the absolute number of photons will vary (blame different LED chips and electrical components needed to generate unique narrow wavelength bands) and the ability of your favorite catalysts to absorb the light changes (blame physics I guess?). And if we wanted to go into that much depth and detail about all that here, it would defeat the purpose of this newsletter being the written version of a clip show.
The reactions we present are as varied as the many wavelengths that are used. We limited ourselves here to 1 cross-coupling reaction although we surely could have done a cross-coupling chemistry of the rainbow feature. You’ll find 2 polymerizations, a cycloaddition, 2 difunctionalizations of olefins, an aziridation, reduction and even an endoperoxide forming reaction. Most of the examples demonstrate reaction methodology development and maximizing catalyst and wavelength optimization. All primarily use the EvoluChem™ LEDs wavelength described for their main reaction in the paper. Many use EvoluChem™ LEDs in conjunction with either the PhotoRedOx Box™, PhotoRedOx Box TC™ or the Lucent360™. The rest are rocking the EvoluChem™ lamp and a clamp approach. On to the chemistry of the Rainbow!
365 nm:
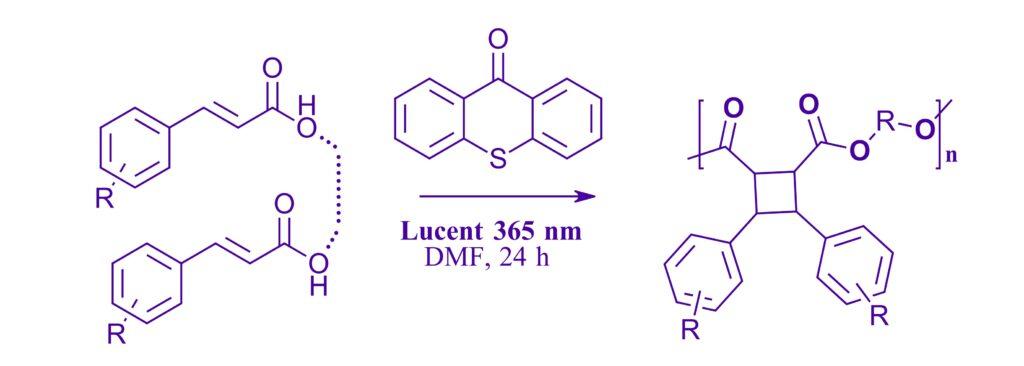
Accessing Cyclobutane Polymers: Overcoming Synthetic Challenges via Efficient Continuous Flow [2+2] Photopolymerization
Sara El-Arid, Jason M. Lenihan, Andrew Jacobsen, Aaron B. Beeler, and Mark W. Grinstaff, ACS Macro Lett. 2024, 13, 607−613.
Note: A 365 nm catalyzed polymerization reaction using thioxanthone as the photocatalyst for generation of a library of novel polymers in the Lucent360™ by Professor Beeler and colleagues at Boston University in both batch and flow.
380 nm:
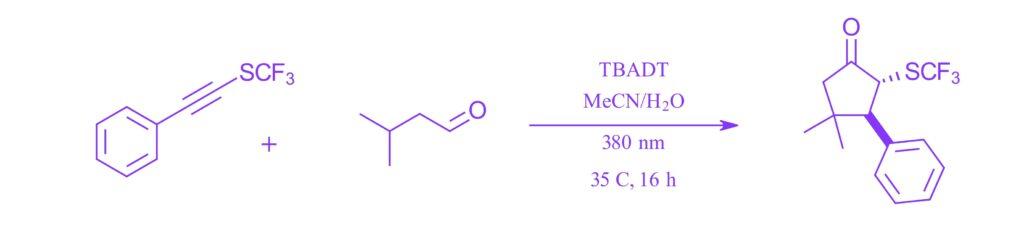
Photocatalyzed Cascade Hydrogen Atom Transfers for Assembly of Multi-Substituted α-SCF3 and α-SCF2 H Cyclopentanones
Nicolas Marie, Jun-An Ma, Vincent Tognetti, and Dominique Cahard, Angew. Chem. Int. Ed. 2024, e202407689.
Note: A general photocatalyzed [3+2] cycloaddition method using tetrabutylammonium decatungstate (TBADT) as the photocatalyst for generating α-SCF3 cyclopentanones from trifluoromethylthioalkynes and aldehydes using the EvoluChem 380nm.
405 nm:
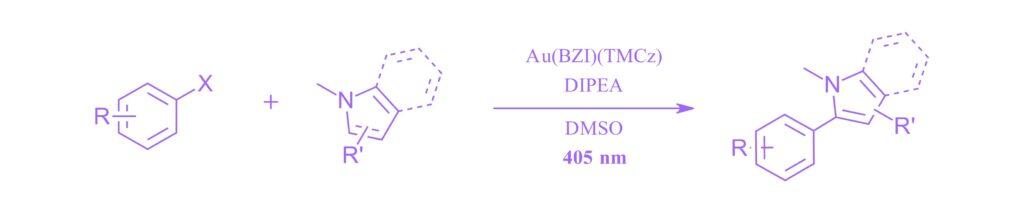
Efficient photoredox catalysis in C–C cross- coupling reactions by two-coordinated Au(I) complex
Byung Hak Jhun, Jihoon Jang, Shinae Lee,Eun Jin Cho and Youngmin You, Nat. Comm. (2024) 15:6586
Note: A catalyst worth its weight in gold from Eun Jin Cho and coworkers at Yonsei University. Low catalyst loadings, high potentials and stable catalysts in this Au catalyzed cross-coupling reaction in the PhotoRedOx Box TC™ with the EvoluChem™ 405 nm LED.
425 nm:
10-Phenylphenothiazine-Organophotocatalyzed Bromo- Perfluoroalkylation of Unactivated Olefins
Koto Tagami, Moeko Nakayama, Tadashi Kanbara, Dominique Cahard, and Tomoko Yajima, J. Org. Chem. 2024, 89, 7084−7094
Note: A visible light bromo perfluoroalkylation from Dominique Cahard and Tomoko Yajima using the EvoluChem™ 425 nm LED. Using 10-phenylphenothiazine (PTH) as the organophotocatalyst the authors were able to synthesize a series perfluoroalkanes at gram scale.
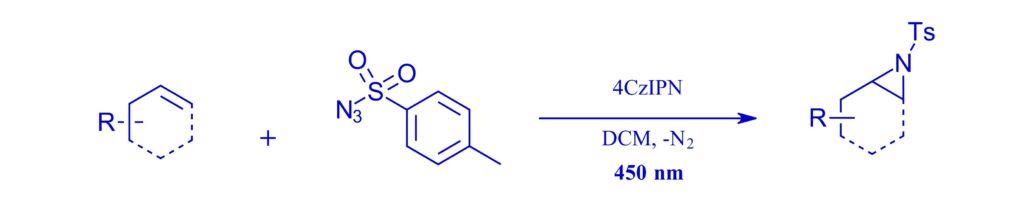
450 nm:
Organic Dye-Sensitized Nitrene Generation: Intermolecular Aziridination of Unactivated Alkenes
Dennis Dam, Nathan R. Lagerweij, Katharina M. Janmaat, Ken Kok, Elisabeth Bouwman, and Jeroen D. C. Codée, J. Org. Chem. 2024, 89, 5, 3251–3258.
Note: An aziridation of a library of alkenes using an organophotocatalyst 4CzIPN in place of transition metals, an EvoluChem™ 450 nm LED and the PhotoRedOx Box™. The reaction was also transferred to work on complex biomolecules.
475 nm:
Photo-induced imine reduction by a photoredox biocatalyst consisting of a pentapeptide
Ryusei Kano, Koji Oohora, Takashi Hayashi, J. Inorg. Biochem. 259 (2024) 112657
Note: Here Takashi Hayashi and coworkers use develop unique peptide bound ruthenium catalysts for imine reductions in aqueous media using an EvoluChem™ 475 nm LED and the PhotoRedOx Box TC™.
525 nm:
Unsymmetrical Anthracene Platforms as Singlet Oxygen Batteries: Effects of Substituents on Photooxygenation and Endoperoxide Thermolysis
Paul De Bonfils, Pierrick Nun, and Vincent Coeffard, Eur. J. Org. Chem. 2024, e202400099
Note: A green light catalyzed method for generating anthracene endoperoxides with Rose Bengal using the EvoluChem™ 525 nm LED and the PhotoRedOx Box™. The authors were able to use this series of endoperoxides to study the substitute effects on the thermal decomposition rates and stability.
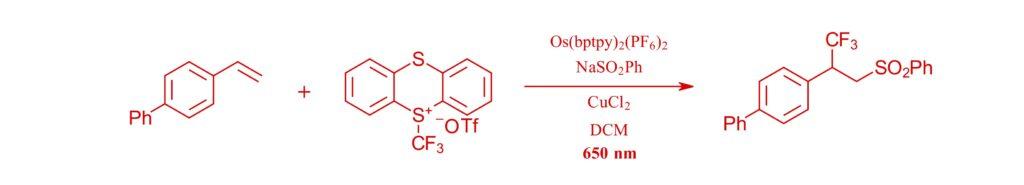
650 nm:
Red-light-mediated copper-catalyzed photoredox catalysis promotes regioselectivity switch in the difunctionalization of alkenes
Tong Zhang, Jabor Rabeah and Shoubhik Das, Nature Communications. (2024) 15:5208.
Note: Shoubhik Das and coworkers used an osmium photocatalyst, an EvoluChem™ 650 nm LED and the PhotoRedOx Box™ to perform this copper co-catalyzed trifluoromethylation/sulfonylation of alkenes.
740 nm:
Robust Miniemulsion PhotoATRP Driven by Red and Near-Infrared Light
Xiaolei Hu, Rongguan Yin, Jaepil Jeong, and Krzysztof Matyjaszewski, J. Am. Chem. Soc. 2024, 146, 19, 13417–13426.
Note: And finally, Krzysztof Matyjaszewski and coworkers at Carnegie Mellon used a water soluble photocatalyst methylene blue, an EvoluChem™ 740 nm LED and the PhotoRedOx Box™ to perform PhotoATRP reactions in emulsions. The NIR 740 nm LED affords deep penetration of light into the emulsions allowing the polymerization with high rates and efficiency.
We hope you enjoyed this selection of papers. Check back with us soon for our first examples using our new 254, 280, 310 and 340 nm LEDs and if you have a reaction to share using the 505, 550 or 808 nm EvoluChem™ LEDs, please share at info@hepatochem.com for the fame and fortune involved in being highlighted in one our future newsletters!