Chemistry is rife with examples of trace metal impurities playing an unwelcome (or occasionally key) role in transition metal catalysis. For every report of a “metal-free” example of a Named Organometallic Reaction there’s an equally likely possibility of something else going on in the system. Copper impurities in iron-catalyzed reactions, nickel found in chromium or palladium-free palladium cross-coupling reactions… we could go on and on (Ref 1). Usually, further investigation uncovers an alternative catalyst for the system which demonstrates remarkable catalyst activity. And so, there’s no reason why photocatalysis should be immune from the scourge of serendipitous, mischievous, or innocuous impurities. This brings us to the paper that we want to discuss this month. In their work, “Unraveling the Prominent Existence of Trace Metals in Photocatalysis: Exploring Iron Impurity Effects” Peng Hu and coworkers discuss a potential minefield in their discovery of a metal-free (and photocatalyst free) photocatalysis reaction for the functionalization of light alkanes (Ref. 2).
Here the authors start with a not so hard to believe initial observation. A metal-free functionalization of an aliphatic C-H bond, initiated by a 365 nm LED, where one could dream and invoke a hydrogen atom transfer mechanism involving a chlorine radical abstraction of a proton (Figure 1). A provocative initial result with 44% conversion for a model system with cyclooctane. A result that would require some explanation of what species is truly absorbing the light and kicking off the reaction. A result that would need rigid examination. Also, a result that the authors doubted from the start, at least once they started looking to modify the chloride source. They just needed to find the how and the why? With a pretty good idea that the how and why was a metal impurity.
Figure 1: “Metal-free” C(sp3)-H functionalization
There are two ways to think about the following paper depending on your point of view or the presence of any deep-seated trauma from your past experiences trying to optimize or reproduce an interesting but unexplainable result.
-
- Chemistry is amazing. Every new observation is an opportunity to learn something unique. Metal catalysts are awesome, awe-inspiring, and magnificent in their ability to perform reactions at ppm or even ppb levels of catalyst loading.
- Chemistry is awful, impossible to ever truly understand. Why does every little detail have to matter so much? Add light to the mix and everything gets worse. I should have gone to art school.
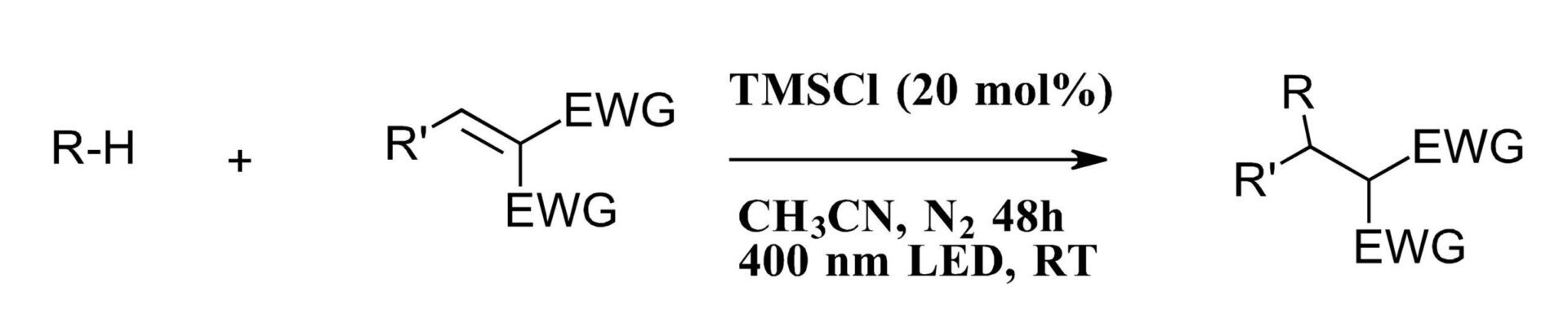
Luckily, the authors dug in and looked at every imaginable detail to better understand what was going on in their system. To start, the authors took care to meticulously limit potential iron or other metal contamination with new glassware, stir bars and reagents while screening chlorine sources for the reaction. TMSCl at 365 nm gave an improved consumption of starting material (>99%) and product formation (55%) while switching this condition to 400 nm gave 98% product. Decreased chloride loading lowered the conversion as did lowering light intensity. Switching to 450 nm turned off the reaction completely (another example of why you should always screen multiple wavelengths). The resulting optimized reaction with TMSCl at 400 nm was suitable for a substrate screen with butane, isobutane and isopropyl. Ethane was successfully transformed at higher pressures and methane with alternate alkenes Figure 2). So, with a “metal-free” system at hand it became imperative to find the metal responsible by studying the reaction mechanism.
Figure 2: Optimized formal “metal-free” system
An investigation of the mechanism demonstrated that both light and a chloride source were necessary for the reaction. Product ratios for substrates such as pentane were consistent with chlorine radical abstraction. Radical scavengers such as TEMPO suppressed the reaction and trapped TEMPO products were observed supporting a radical mechanism. However, no species existed in the system suitable for absorption of the 400 nm to initiate direct interaction between the substrates and the chloride. They tested the reaction with added iron, copper and cerium chloride salts as additives with iron giving the highest conversion of the three.
A rigorous study was then conducted to find the source of the known unknown metal contamination. All chloride sources and reagents were tested by ICP-MS for Fe, Cu, Ce and Ni. Analysis of the individual reagents and substrates found 10 ppb to 6 ppm metals present (If you are curious about metal levels in reagents from various suppliers, check out the Supporting Info). Not enough to account for such high conversation in the reaction system or suggest the bad actor. However, analysis of the reaction mixture themselves found iron and copper at levels equivalent to 10-4 mol% catalyst. With the most likely source of minimal contamination being a combination of all reagents and metal from gas delivery systems or simply the ambient environment. Ultimately, through extensive studies with 10-4 mol % FeCl3 added to the system, an experimental condition was found that increased rate of reaction compared to ambient iron levels. The role of trace metal in the system was further confirmed by addition of pyridine-based ligands to the system to complex iron leading to decreased yields.
Extensive studies were untaken to complete the full picture of the reaction. Including studying the reaction with multiple vial and stir bar sources, sizes and styles, different flasks made of quartz or PTFE with minimal difference in product conversion. The experiment was repeated in two additional laboratories in different locations. So, it would seem that there is always enough iron around to catalyze the reaction. Ultimately, the authors determined that even with the best care, it was impossible to limit exposure of the system to iron in a standard laboratory environment to a level low enough to not influence catalysis. And since catalysis is the goal, well it is what it is. A formally metal-free, photocatalyst-free reaction for functional alkanes. What the paper does do however is present a vary convincing case for everyone to consider just how innocent iron might be in their reaction of choice. Or next time that a reaction works better after sparging with nitrogen compared to air, a consideration of whether you are adding beneficial iron to your system.
References:
ACS Catal. 2022, 12, 6, 3644–3650. https://doi.org/10.1021/acscatal.2c00967
Yahao Huang, Miao Wang, Wei Liu, Qiang Wu, and Peng Hu, The Journal of Organic Chemistry 2024 89 (6), 4156-4164. DOI: 10.1021/acs.joc.4c00155