The attack of the photocatalytic microrobots!
 We have intended to write a bit about visible-light decomposition of contaminants for a while… so what better entry into the topic than a story about self-propelled autonomous microrobots that can swim through mazes to seek and destroy microplastics?
We have intended to write a bit about visible-light decomposition of contaminants for a while… so what better entry into the topic than a story about self-propelled autonomous microrobots that can swim through mazes to seek and destroy microplastics?
#Mircrorobots #Prey on #Microplastics – see our paper by @SMohsenBM in @ACS_AMI ‘A Maze in Plastic Wastes: Autonomous Motile Photocatalytic Microrobots against Microplastics’ https://t.co/spOprW1OFY pic.twitter.com/qUQPX3KOJF
– Pumera Research Group (@PumeraGroup) May 19, 2021
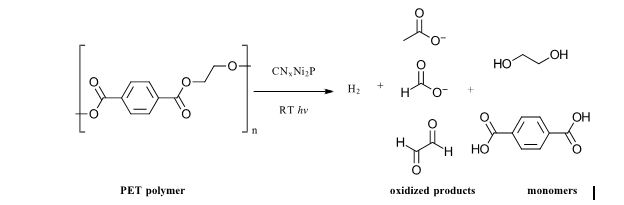
At issue for the present work are microplastics, defined as less than 5 mm large pieces of plastic that have broken off from larger plastics from either thermal, mechanical or photochemical stress or as a result of their intentional addition to products as fibers in clothing or additives to personal care products like cosmetics and detergents. (To note, this is a different scenario than small molecule plastic additives like bisphenols and phthalates. (Also a major environmental problem) The authors cite a yearly global production rate of ~380 million metric tons per year of plastics, which is a number so large that it is impossible to be put into any sort of real context. And it’s increasing. In the environment, the low solubility/hydrophobic nature of microplastics can cause the accumulation of organic pollutants, heavy metals and pathogens on their surface. They are too small for removal by standard remediation methods for plastic waste (think fishing nets) and small enough that they are easily consumed by aquatic species. Microplastics maintain most of the stability and low biodegradability we expect from plastics, so they are also with us for a long time. As a result, microplastics have been found pervasively in pristine environments, tap water and many food products. So much so, that studies tracking their prevalence can easily be contaminated at each step of the collection and analytical process by microplastics in airborne particles, chemical reagents and filtered lab water among other sources. As a result, the full extent of their reach is not well-understood. Many thermal, chemical or photocatalytic degradation methods are in use or under development for water purification, degradation of small molecules, persistent pollutants and plastics. However, light (specifically sun-light) driven methods offer the most energy efficient possibilities. For mixed plastic wastes, a recent example uses an inexpensive non-toxic carbon nitride/nickel phosphide photocatalyst to reform polyethylene terephthalate (PET) and polylactic acid (PLA) polymer waste into H2 and other chemicals. (Ref 2) Figure 1: Example of Photocatalytic degradation of PET polymer from Ref 2
 However, most similar technologies work best on larger pieces of plastic. For the degradation of the microplastics, their small size, relatively chemically inert surface and low solubility make interactions with the photocatalyst difficult to achieve without extensive stirring (energy demanding) or pretreating the surface with deposition of catalyst (not applicable for real world applications). As we all are familiar, photoredox catalysis offers a wide range of solutions to take advantage of reactive species that can be generated with visible light. However, we probably don’t want to dump a bucket of most of the photocatalysts that we use on a daily basis in the ocean (for a variety of reasons, toxicity, cost, solubility, lack of recovery). For that we have photocatalytic microrobots. For the task at hand, the authors turn to their microrobots army (Ref 3). Previously, the authors have described a visible light-driven particle based on BiVO4 (Ref 4) The irregular star-shaped self-motile photocatalysts (4-8 μm) can under irradiation with sunlight in the presence of dilute 0.1% wt solutions of H2O2 achieve speeds between up to 6 μm/s (Figure 2A). The robots are able to move, based on what the authors describe as “asymmetrical generation of chemical species on the surface of multifaceted BIVO4 motors induced by light illumination.” (Ref 4) Mainly, the photoexcited electrons react with oxygen or H2O2 to generate reactive oxygen species or water. The electron holes can oxidize water to hydroxyl radical or reduce H2O2. The asymmetric shape of the particles causes these reactions at different rates, which creates a local electric field and causes charge particles to move forward. A video of robots in motion can be seen here: xxxx. For this work, the particles can be magnetized by addition of Fe3O4 nanoparticles on the surface for recovery or as an additional method to induce motion. Figure 2: Scheme demonstrating the motility of BiVO4/ Fe3O4 microrobots (modified from Ref 1)
However, most similar technologies work best on larger pieces of plastic. For the degradation of the microplastics, their small size, relatively chemically inert surface and low solubility make interactions with the photocatalyst difficult to achieve without extensive stirring (energy demanding) or pretreating the surface with deposition of catalyst (not applicable for real world applications). As we all are familiar, photoredox catalysis offers a wide range of solutions to take advantage of reactive species that can be generated with visible light. However, we probably don’t want to dump a bucket of most of the photocatalysts that we use on a daily basis in the ocean (for a variety of reasons, toxicity, cost, solubility, lack of recovery). For that we have photocatalytic microrobots. For the task at hand, the authors turn to their microrobots army (Ref 3). Previously, the authors have described a visible light-driven particle based on BiVO4 (Ref 4) The irregular star-shaped self-motile photocatalysts (4-8 μm) can under irradiation with sunlight in the presence of dilute 0.1% wt solutions of H2O2 achieve speeds between up to 6 μm/s (Figure 2A). The robots are able to move, based on what the authors describe as “asymmetrical generation of chemical species on the surface of multifaceted BIVO4 motors induced by light illumination.” (Ref 4) Mainly, the photoexcited electrons react with oxygen or H2O2 to generate reactive oxygen species or water. The electron holes can oxidize water to hydroxyl radical or reduce H2O2. The asymmetric shape of the particles causes these reactions at different rates, which creates a local electric field and causes charge particles to move forward. A video of robots in motion can be seen here: xxxx. For this work, the particles can be magnetized by addition of Fe3O4 nanoparticles on the surface for recovery or as an additional method to induce motion. Figure 2: Scheme demonstrating the motility of BiVO4/ Fe3O4 microrobots (modified from Ref 1) 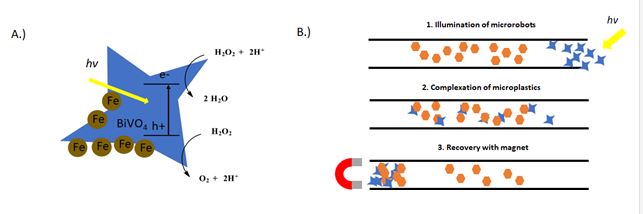 Both the motion and the stirring caused by the particles during motion can increase contacts with the microplastics. Their irregular shapes allow them to attach to polymers of many different shapes and sizes. This is demonstrated by the adsorption of the microrobots onto microplastic pieces of polylactic acid (PLA) (72% coverage), polycaprolactone (PCL) (44%), polyethylene terephthalate (PET) (66%) and polypropylene PP (27%). For the experiment, a dish containing microrobots and dilute H2O2 was illuminated with visible light for 3 h. Samples were then dried and washed and the coverage was determined. Controls with immobile microbots (no illumination) show no deposition. The recovery of microplastics was demonstrated in an experiment using 5-10 cm long channels containing microplastics of various polymers (Figure 2B). In the presence of light and H2O2, the microrobots move in a random dispersion through the channel interacting with the microplastic pieces becoming absorbed. In the presence of a magnetic field, the microplastic-microrobot adducts can be recovered on one side of the channel in similar recoveries as observed for the dispersion experiment discussed above. The robots even swim a maze to find the plastics. The ability of the microrobots to degrade the microplastics was also investigated. The plastic pieces gradually lost weight over the course of visible light illumination in the presence of the microrobots. A 3% weight loss of individual microplastics pieces after 7 days was observed. The increase of hydrophilicity due to addition of oxygen functional groups to the microplastics was demonstrated by wetting angle and by X-Ray spectroscopy where the surface of the plastics were dramatically changed. So, in this proof of concept, the authors have demonstrated the both the capture, recovery and beginnings of plastic degradation in one system. There is certainly more work to come from this technology. Check out the paper for more interesting figures, videos and discussion on the future of using microrobots for remediation. And for something slightly different, here is video from Pumera’s robot army cleaning up uranium waste from 2019. All in all, just a really fun work in a field we know very little about and that we hope you enjoy as much as we did. In more photoredox catalysis related news, a few papers caught our eye this month. First, is a work by the Molander and Gutierrez labs on a open air, catalyst free trifluoromethylthiolation reaction.
Both the motion and the stirring caused by the particles during motion can increase contacts with the microplastics. Their irregular shapes allow them to attach to polymers of many different shapes and sizes. This is demonstrated by the adsorption of the microrobots onto microplastic pieces of polylactic acid (PLA) (72% coverage), polycaprolactone (PCL) (44%), polyethylene terephthalate (PET) (66%) and polypropylene PP (27%). For the experiment, a dish containing microrobots and dilute H2O2 was illuminated with visible light for 3 h. Samples were then dried and washed and the coverage was determined. Controls with immobile microbots (no illumination) show no deposition. The recovery of microplastics was demonstrated in an experiment using 5-10 cm long channels containing microplastics of various polymers (Figure 2B). In the presence of light and H2O2, the microrobots move in a random dispersion through the channel interacting with the microplastic pieces becoming absorbed. In the presence of a magnetic field, the microplastic-microrobot adducts can be recovered on one side of the channel in similar recoveries as observed for the dispersion experiment discussed above. The robots even swim a maze to find the plastics. The ability of the microrobots to degrade the microplastics was also investigated. The plastic pieces gradually lost weight over the course of visible light illumination in the presence of the microrobots. A 3% weight loss of individual microplastics pieces after 7 days was observed. The increase of hydrophilicity due to addition of oxygen functional groups to the microplastics was demonstrated by wetting angle and by X-Ray spectroscopy where the surface of the plastics were dramatically changed. So, in this proof of concept, the authors have demonstrated the both the capture, recovery and beginnings of plastic degradation in one system. There is certainly more work to come from this technology. Check out the paper for more interesting figures, videos and discussion on the future of using microrobots for remediation. And for something slightly different, here is video from Pumera’s robot army cleaning up uranium waste from 2019. All in all, just a really fun work in a field we know very little about and that we hope you enjoy as much as we did. In more photoredox catalysis related news, a few papers caught our eye this month. First, is a work by the Molander and Gutierrez labs on a open air, catalyst free trifluoromethylthiolation reaction.
Check out this catalyst”free, open-to-air #trifluoromethylthiolation. Through an incredible collaboration with @gutierrez_lab, we examined single-electron transfer events promoted by electron donor-acceptor (EDA) complex photoactivation. Read it here https://t.co/ItWqJ4zd3v pic.twitter.com/9nzBeYQsnp – Molander Group (@molandergroup) May 21, 2021
Second, Melchiorre and coworkers shine light on a well-established iridium catalyst (not quite what you are going to expect) to enable enantioselective C-C couplings.
link here : https://t.co/PP2oYSKskV@GEMCrisenza @adriana_faraone @EugenioGandolfo @DMZ_MazDa
– Melchiorre Group (@MelchiorreGroup) May 24, 2021
References:
- Beladi-mousavi, S. M.; Hermanova, S.; Ying, Y.; Plutnar, J.; Pumera, M. A Maze in Plastic Wastes: Autonomous Motile Photocatalytic Microrobots against Microplastics. Mater. Interfaces 2021. https://doi.org/10.1021/acsami.1c04559.
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. Am. Chem. Soc. 2019, 141 (38), 15201–15210. https://doi.org/10.1021/jacs.9b06872.
- Wang, H.; Pumera, M. Coordinated behaviors of artificial micro/nanomachines: from mutual interactions to interactions with the environment. Chem. Soc. Rev. 2020, 49, 3211-3230.
- Villa, K.; NovotnyÌ, F.; Zelenka, J.; Browne, M. P.; Ruml, T.; Pumera, M. Visible-Light-Driven Single-Component BiVO4 Micro- motors with the Autonomous Ability for Capturing Microorganisms. ACS Nano 2019, 13, 8135-8145.

