Near-Infrared (NIR) Light Gaining Interest
For most of the previous century, photochemistry invoked images of high-powered mercury lamps, intense UV light and classical reactions such as [2+2]-cycloadditions, cyclizations and radical rearrangements (Ref 1). This changed more than ten years ago with the rediscovery of photoredox chemistry and the increased availability of LEDs (Ref 2). Irradiation of common photocatalysts such as ruthenium and iridium with visible light from blue LEDs (450-470 nm) afforded highly oxidative and reductive photocatalysts able to activate difficult organometallic cross-coupling reactions. Blue LEDs (450 nm) and photoreactors are now common in many synthetic labs with seemingly unlimited applications in organic synthesis (Ref 3). Now, a number of red light applications in photochemistry are expanding the options available to synthetic organic chemists. This post provides an overview of many of these red light applications.
Osmium Photocatalysts
Several recent papers (see footnotes) further expand the tool kit for synthetic chemists to the Near-IR. The Rovis group at Columbia University and BMS recently published an exciting preprint using red LEDs (Ref 4). Low energy red LED (740 nm) can activate Os(II) photosensitizers to perform several of the same reactions previously demonstrated by blue LEDs but with several potential advantages. The advantages derive from two key differences between the common metal photoredox catalysts – like Ru(II) and Ir(III) -with Os(II) systems (see Figure 1). For Ru(II) and Ir(III) systems, the high extinction coefficients of the photocatalyst prevent light penetration deep into a reaction medium. This limits the amount of light available into the reaction volume and requires modification of the reaction when scaling up photoredox. Often the concentration of the catalyst is lowered for batch reactions (Ref 5) or the reaction is performed in flow (Ref 6). Secondly, in these Ir/Ru systems the ground S0is excited through a metal-ligand charge transfer band (MLCT) to the excited state S1. It then must inter-system cross to give the T1 species. This decay is both inefficient, the quantum yield for common Ru photocatalyst is ~9%, and results in the loss of ~25% of the light energy thermally necessitating high-energy light.
Figure 1: Comparing traditional metal photoredox with Near-IR Os(II) systems (Figure adapted from Ref 4 and references within)
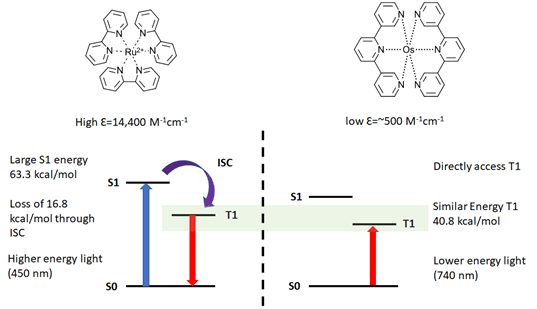
Os(II) photosensitizers can directly access the excited triplet state with NIR radiation (S0↔T1) converting NIR light into chemical energy with minimal loss. The resulting Os(II) T1 state is of similar energy as found in the Ir and Ru systems (40.8 vs. 46.5 kcal/mol). In the Rovis paper, the group demonstrates photoredox, photopolymerization, and metallaphotoredox reactions as well as a mole scale arene trifluoromethylation in batch. All excellent examples of red light applications in photochemistry. And because of the lower extinction coefficient, red light can penetrate 10x farther into the reaction medium with an observed advantage in scaling up photoredox chemistry.
Figure 2: Batch Scale 1L trifluoromethylation reaction with Osmium photocatalyst
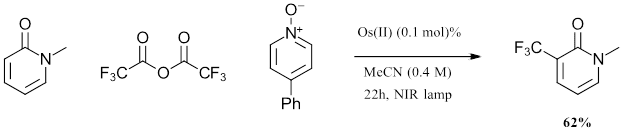
In comparing the 1L batch scale up for the Osmium trifluoromethylation scale with the reported ruthenium trifluoromethylation reaction, several key features are observed. At 1L scale, the authors observed a 62% yield after 22 hours with eight 740 nm lights. In this setup, the red light penetrates 23x further into the reaction solution than the blue light. With the osmium, the reaction yield increased by 31.6% with larger scale, while for the ruthenium system the yield lowered 27.5% as scale increased. This demonstration shows advantages that can be achieved in scale up with osmium chemistry.
However, we should point out the success in scaling up iridium photochemistry to large scales reported by Harper, et. al. with predictable experimental control of reaction parameters such as lowering catalyst concentration and light intensity (Ref 5). While not a straight forward translation of the small-scale reactions, photoredox reactions have been performed on an industrial scale leveraging 365-450nm light sources and traditional catalysts. Additionally, osmium itself is problematic due to cost and toxicity concerns, but the method outlined here could be expanded and utilized with the synthesis of other novel photocatalysts.
Triplet Fusion Upconversion
Triplet fusion upconversion is the process where two lower energy photons are converted into one higher energy photon. This process is often utilized in photovoltaics or imaging. Another application of red light in photochemistry is enabling the generation of higher energy photons through this process.
The Rovis Group previously described a system to use Red LED to initiate photoredox catalysis through a process known as triplet fusion upconversion (Ref 7). In this work, the authors access orange and blue light from low energy infrared light by matching the appropriate sensitizer and an annihilator, generating a highly oxidative/reductive photocatalyst normally accessible only through blue light (Figure 3). In this system, the sensitizer absorbs one photon of light to generate the excited species and decays to give a triple excited sensitizer. One triplet can react with one annihilator to give the excited annihilator. Two annihilators can combine their energy to a higher excited state, with subsequent fluorescence to release of one higher energy photon from one of the annihilators.
This new higher energy photon can activate a photocatalyst (for example Ru or Ir) to initiate a photoredox catalytic cycle. The authors describe this process as generating a magnitude of “light bulbs inside the flask”. This enables the transfer of light/energy for catalysis into biological systems or through photoactive polymers or material) where blue light cannot penetrate but higher energy light than red is needed to activate the reaction.
Figure 3: Electron description of triplet fusion upconversion. Adapted from Ref. 9
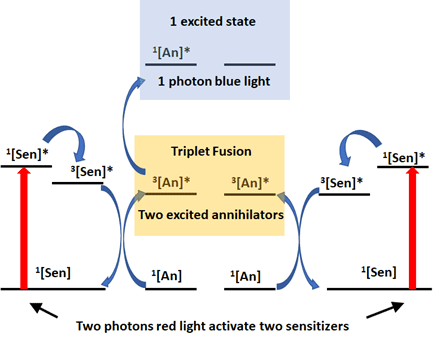
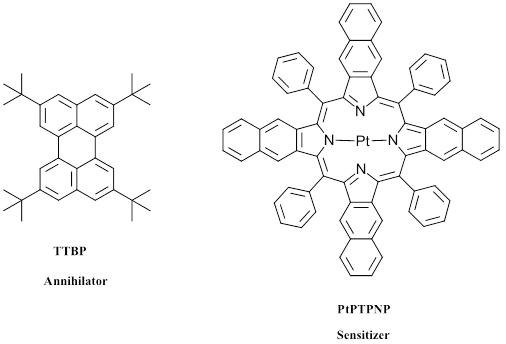
Figure 4: Example of a sensitizer/ annihilator pair for emission of 1 photon blue light
Click Chemistry
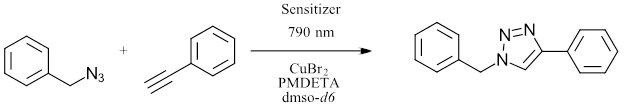
Yet another example of a red light application in photochemistry is the ability of red LEDs to initiate one of the most commonly used reactions in bioorganic chemistry. Stremhel and coworkers demonstrated an NIR initiated Cu azide-alkyne click reaction (Ref 8). Using a series of cyanine photosensitizers the group was able to catalyze the reduction of Cu(II) to Cu (I) using red LED (790 nm) under ambient temperature (Figure 5). The method accesses the catalytically active Cu(I) species without an additional reducing agent. In this system, the Cu can be used at ppm levels. This work enabled the synthesis of several block polymers and represents a system able to perform click reactions where desired monomers might absorb in UV or visible region or deep in biological tissue.
Figure 5: Click Reaction
Red Light in Cancer Therapeutics
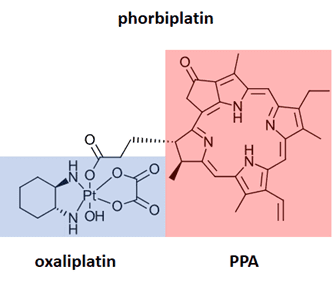
Methods for performing NIR chemistry in vivo are of significant interest for photodynamic therapy. The therapeutic window for most photodynamic therapy lies between 600-800 nm, where the energy of each photon is high enough to activate a photosensitizer but low enough to achieve penetration through biological tissue. A demonstration of the use of red LED in vivo is shown in the recent Pt(IV) prodrug study demonstrated by Zhu Guangyu and coworkers (Ref 9). His team developed phorbiplatin, an anti-cancer prodrug activated by red light. Phorbiplatin is inactive in the dark, but upon irradiation with red light (650 nm) is converted to oxaliplatin, an approved anticancer drug and pyropheophorbide, also known to kill tumor cells. This onsite activation of the prodrug minimizes damage to healthy cells. In ten minutes of irradiation with low power 650 nm, 7 mW/cm2, 81% of the phorbiplatin is converted to the oxaliplatin and pyropheophorbide. The selectivity of the prodrug can also lead to significant improvement in activity. For example, phorbiplatin showed improved treatment for in cells and mouse tumors compared to treatment with oxiplatin.
As demonstrated by the phorbiplatin prodrug example, chemistry that can occur within the therapeutic window has great promise for a broad number of applications both in medicine and materials. Whether it is catalysts that can perform chemistry with NIR such as the Cu or Os examples above, or methods for generating higher energy light from red light, expanding this field can have great promise.
Figure 6: Phorbiplatin
References:
(1) Hoffman, Norbert, “Photochemical Reactions as Key Steps in Organic Synthesis” Chem. Rev. 2008, 108, 1052-1103. https://pubs.acs.org/doi/10.1021/cr0680336
(2) Jagan M. R. Narayanam, Corey R.J. Stephenson, “Visible Light Photoredox Catalysis: Applications in Organic Synthesis” Chem. Soc. Rev. 2011, 40, 102-113. https://doi.org/10.1039/B913880N
(3) Leyre Marzo, Santhosh K. Pagire, Oliver Reiser and Burkhard König, “Visible-Light Photocatalysis: Does it make a difference in Organic Synthesis, Angew. Chem. Int. Ed., 2018, 57, 10034-10072. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201709766
(4) Benjamin D. Ravetz, Nicholas E. S. Tay, Candice L. Joe, Melda Sezen-Edmonds, Michael A. Schmidt, Yichen Tan, Jacob M. Janey, Martin D. Eastgate, Tomislav Rovis, “Spin-Forbidden Excitation Enables Infrared Photoredox Catalysis” ChemRxiv, 2020.
(5) Harper, K. C.; Moschetta, E. G.; Bordawekar, S. V.; Wittenberger, S. J. “A laser driven flow chemistry platform for scaling photochemical reactions with visible light.” ACS Cent. Sci. 2019, 5, 109-115. https://pubs.acs.org/doi/10.1021/acscentsci.8b00728
(6) Thomas H. Rehm, “Reactor Technology Concepts for Flow Photochemistry” ChemPhotoChem, 2020, 4, 235-254. https://doi.org/10.1002/cptc.201900247
(7) Ravetz, B. D.; Pun, A. B.; Churchill, E. M; Congreve, D. N.; Rovis, T.; Campos, L. M. “Photoredox catalysis using infrared light via triplet fusion upconversion” Nature 2019, 570, 343-346. https://www.nature.com/articles/s41586-018-0835-2
(8) Kütahya, C.; Yagci, Y.; Strehmel, B. “Near-infrared photoinduced copper-catalyzed azide-alkyne click chemistry with a cyanine comprising a barbiturate group” ChemPhotoChem 2019, 3, 1180-1186. https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cptc.201900012
(9) Wang Z., Wang N., Cheng S.C., Hirao H., Ko C.C., Zhu G. “Phorbiplatin, a Highly Potent Pt(IV) Antitumor Prodrug That Can Be Controllably Activated by Red Light” Chem, 2019, 5 (12) P3151-3165. https://doi.org/10.1016/j.chempr.2019.08.021

