This month we ponder the question, should you pulse the light source in your photocatalysis reaction? A weird and fun question, and weird and fun questions are why we started this little blog in the first place. We’re inspired by this recent paper entitled “Investigating the Effects of Pulsed LED Irradiation in Photoredox Catalysis: A Pilot Study” by Timothy Connell and Alex Bissember and coworkers (Ref 1) and motivated to write about it due to its criminally low number of article views as of the time of this post. Can you influence a reaction by turning on and off the light? Of course. Also, pulsed irradiation is known to affect the production of phytochemical in plants. So, do we think pulsed LED irradiation will have broad implications for synthetic photochemistry? For that, we have no idea, because that question isn’t answerable yet. But should you try? Maybe. Let’s discuss.
“What would happen if….”
A question many of us have asked at the bench regarding some seemingly trivial reaction detail that might not be so trivial at all. Like the size of a flask or stir bar, the heat source or the rate and order of addition of reagents among many, many other things not listed when you write out the chemical equation.
“This photocatalysis reaction isn’t working as well as I would like. What would happen if I just turned my LED off and turned it back on again.” Hey, it works for computers. We’ve all seen the 5-minute LED on, 5-minute off plots to prove that a reaction is light driven. But what about on/off every minute, every second, 1000 times per second? Where’s the limit? Sometimes, an idea is exceedingly simple and still incredibly complex.
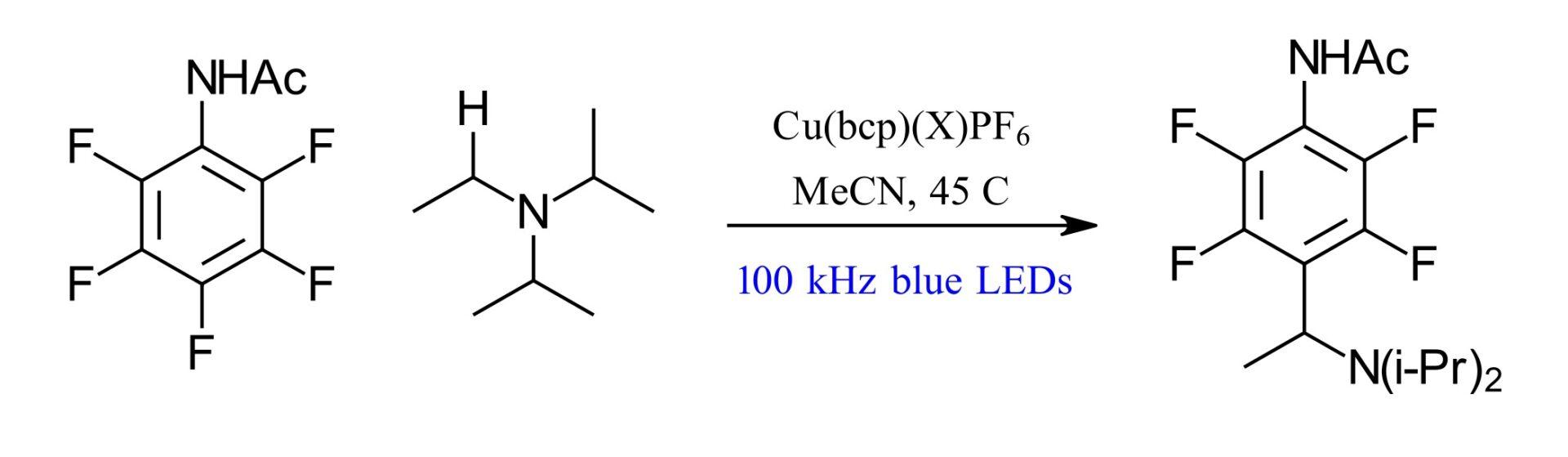
The authors of the paper in question had their “what would happen if” moment in a 2018 publication using a blue LED copper-catalyze C-F functionalization reaction. They observed a sluggish reaction that showed an increase in efficiency from 50% to 87% with pulsed irradiation compared to standard irradiation. Additionally, the optimal 100 kHz pulsing “approximately matched the excited state lifetime of copper catalyst” (Ref 2). So, pulsed LED photochemistry. Is this going to be a thing? Is this a great idea, or even an interesting idea or just an odd one. For that we need data.
Figure 1: Initial result with Pulsed LED irradiation (Ref 2)
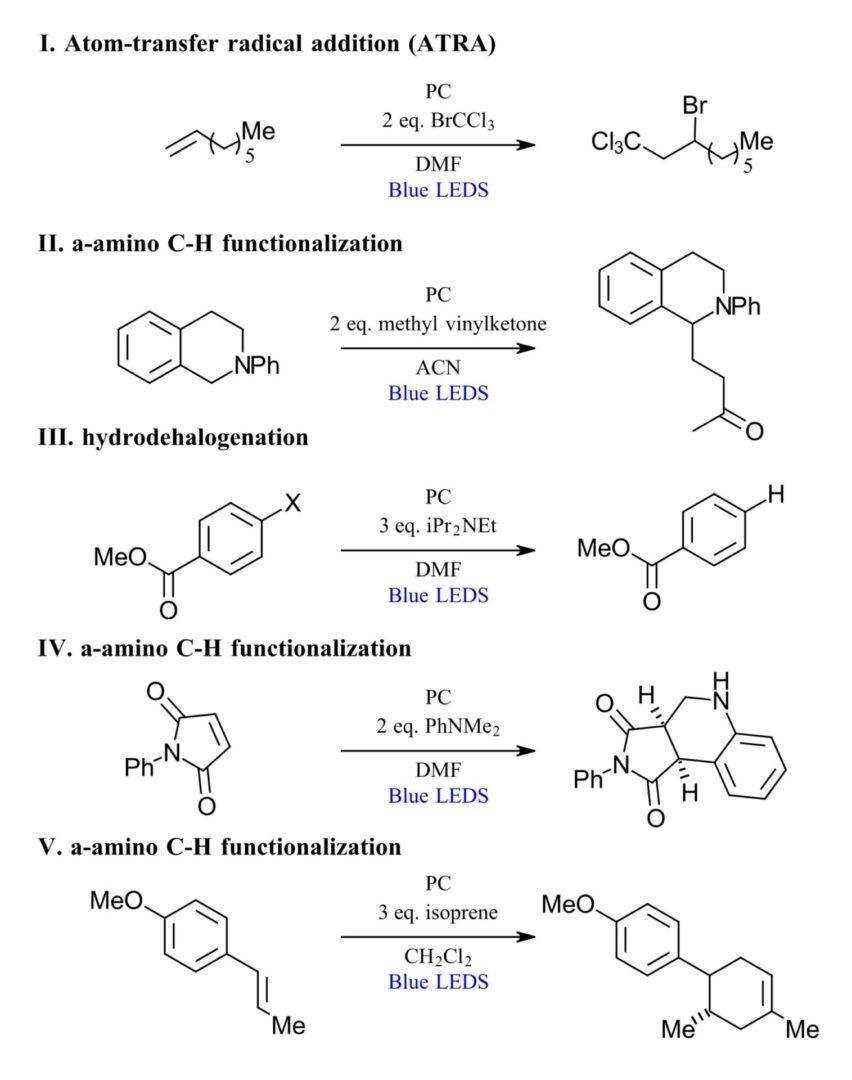
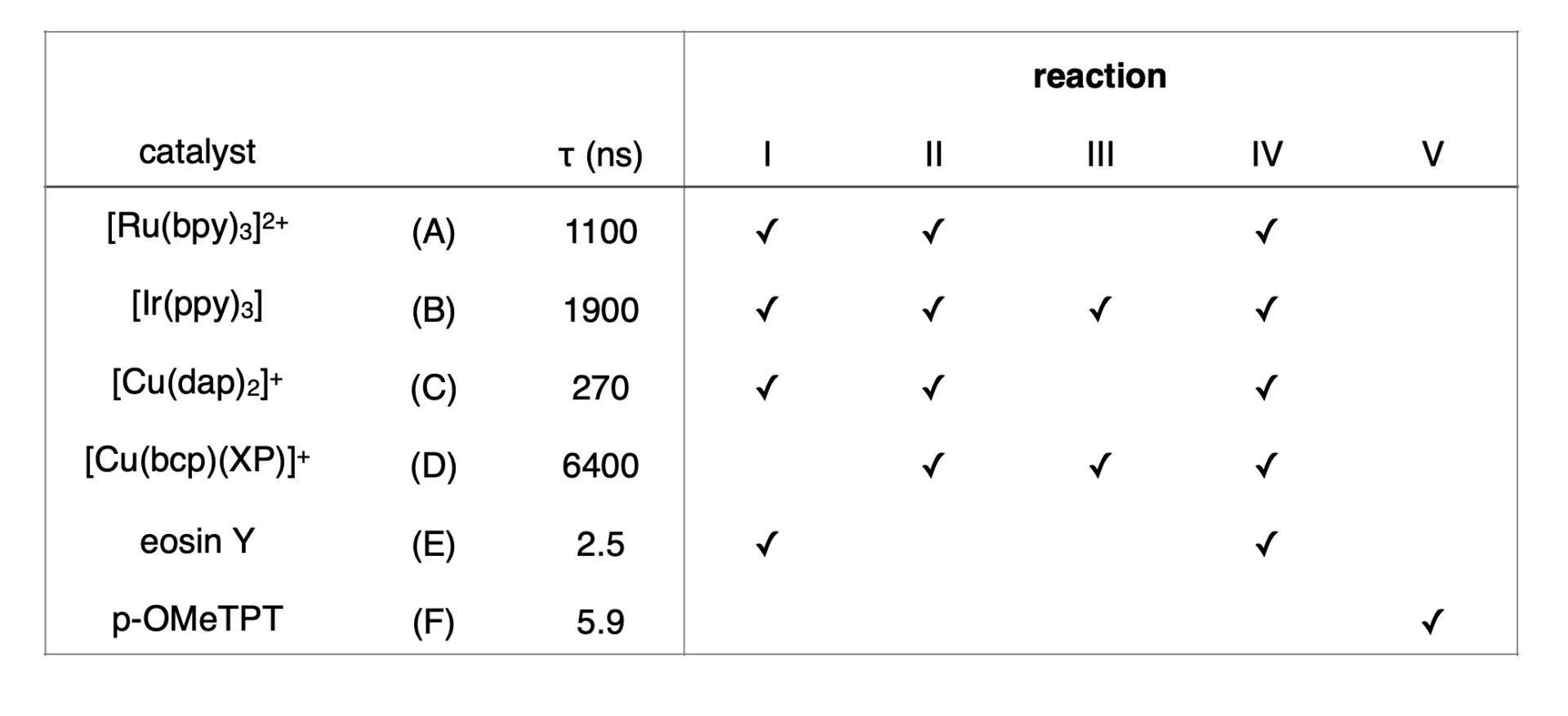
In present work, the authors chose 5 reactions to test the effect of continuous, 10 kHz pulsed and 100 kHz pulsed irradiation. The reactions were selected to represent different classes of reactions as shown in Figure 2 including an atom-transfer radical addition (ATRA), α-amino C-H functionalization, hydrodehalogenation and a radical cation Diels-Alder cycloaddition. Each reaction was tested with 6 photocatalysts representing well-established photocatalyst classes including ruthenium [Ru(bpy)3]2+, iridium [Ir(ppy)3] and copper [Cu(dap)2]+ and [Cu(bcp)(XP)]+ along with organic photocatalysts eosin Y and pOMeTPT. The excited states (τ) of the catalysts ranged from 2.5 to 6400 ns. Each reaction was monitored for 24 hours, with product quantified by GC in duplicate.
Figure 2: Selected reactions for testing LED pulsing (Adapted from Ref 1, Figure 2)
With 5 reactions and 6 catalysts, 3 light regiments (continuous, 10 kHZ and 100 kHz) and multiple time points, there is a lot of data. And many, many plots. Not all the catalysts successfully performed each of the reactions, so the reactions that gave product are denoted in Table 1. And to expect one giant magic trend was unrealistic (And I’m sure the authors didn’t either). This is really the type of paper that is worth digging into deeply for those of you who want to read the whole article. So, what stood out to us? What can we learn by breaking down the different reaction classes?
Table 1: Summary of reactions, catalysts and excited state lifetimes (Adapted from Ref 2)
Some of the reactions work incredibly well (ATRP – Reaction I), catalyst A, B and E gave nearly 99% conversion by 24 hours for continuous, 10 kHz and 100 kHz regimes leaving little room for improvement. Catalyst C gave only 36% conversion with continuous irradiation, but pulsing at either 10 kHz or 100 kHz was even lower (21% and 29%). The reaction profile for Catalyst A and B shows little difference for time, however, for catalyst E, the initial reaction profile shows an increase for kHz irradiation compared to continuous or 100 kHz. For Reaction II, little difference was observed for the reaction profiles between catalysts A-D, although at 24 hours Ru catalyst A showed an improvement from 90 to 100% conversion with pulsed irradiation at 100 kHz compared to continuous.
For Reaction III, only catalyst B and D had sufficient energy to perform deiodination and debromination. Interestingly, pulsed irradiation showed no difference in reaction profile for irradiation regime for the deiodination with catalyst D, however, debromination conversion demonstrated a clear decrease with pulsed light (continuous > 100 kHz > 10 kHz).
The most consistent improvement for pulsed light was observed for Reaction IV, where catalyst A, B and D showed significant increase in efficiency for pulsed light sequences. At the slowest pulse 10 kHz, catalyst B and D show greater than 10% improvement than continuous irradiation after 5 hours. This trend followed with the lifetime of the catalyst D >B>A. Reaction V, the only reaction studied proposed to be a radical chain mechanism, also showed increased reactivity with the slowest pulsed reaction for catalyst E.
What does this all mean? The authors can sum up their own thoughts much more gracefully than we can and do a great discussion on each reaction and mechanism on what can happen with pulsed light (as best as can be) when no grand trend exists. And we suggest that you read their words as they go into detail on this work. In their words…
“The results of this pilot study indicate that pulsed LED irradiation can influence the progress of photoredox-catalyzed reactions positively, negatively or negligibly…often differ across reaction classes, may vary across substrates within a specific reaction and may different depending on the identity of the photoredox catalyst”
Well, the authors original “what would happen if…” observation that the lifetime of their catalyst and pulse frequency were similar didn’t really hold up to further inspection in this work. But they still observed significant effects on reactions due to pulsed light and found a class of reaction (Reaction IV) that showed a general trend of improvement with pulsed light.
We like to think our photochemical reactions always make sense. But we’ve also seen 2% product in the absence of light, or a product that slowly goes away at the end of your reaction as you try to push it. Or that little bit of brownish/black sludge on the side of your vial. And so yes, there are things going on in our photocatalysis reactions that sometimes don’t make a lot of sense when writing up a perfect mechanism. Sometimes, chemistry is messy. Most of our reactions only rely on light for one step of the process and have several (or many) downstream steps or intermediates that could be affected by light positively or negatively. Most of our reactions have plenty of light (except those that don’t), the light is absorbed by our photocatalyst and not our substrates (except when it is) and our reaction intermediates and product don’t interact with the light either (often unknown). All of which seem like great reasons to try to vary and pulse the light in a reaction, maybe not from a theoretical sense but when from a practical sense for a difficult reaction or one at scale.
We’re interested in reading more on this topic, so if you have an example of pulsed light or something we should read next, send it to us at info@hepatochem.com or on our new account on Bluesky@EvoluChem.com.
Thanks for reading.
References:
(1) Burt, L. K.; Robertson, J. C.; Breadmore, M. C.; Connell, T. U.; Bissember, A. C. Investigating the Effects of Pulsed LED Irradiation in Photoredox Catalysis: A Pilot Study. Organometallics 2024, ASAP. https://doi.org/10.1021/acs.organomet.4c00232.
(2) Nicholls, T. P.; Robertson, J. C.; Gardiner, M. G.; Bissember, A. C. “Identifying the potential of pulsed LED irradiation in synthesis: copper-photocatalysed C−F functionalisation.” Chem. Commun. 2018, 54, 4589−4592. https://pubs.rsc.org/en/content/articlelanding/2018/cc/c8cc02244e