Spring is nearly here in Massachusetts. The snow has almost completely melted, and the days are getting longer. Soon the first flowers will bloom and some of those flowers are catalysts for photoredox cross-coupling reactions. Wait, what? File this work that appeared recently in the RSC Green Chemistry (open access) under “Things we would never think to try” and “Why we love science”. It’s a fascinating example of photoredox chemistry with organic dyes.
Spring is nearly here in Massachusetts. The snow has almost completely melted, and the days are getting longer. Soon the first flowers will bloom and some of those flowers are catalysts for photoredox cross-coupling reactions. Wait, what? File this work that appeared recently in the RSC Green Chemistry (open access) under “Things we would never think to try” and “Why we love science”. It’s a fascinating example of photoredox chemistry with organic dyes.
Think about the last reaction that you ran. Perhaps you dried a solvent, distilled a reagent or sparged to remove oxygen. If it is a photoredox reaction hopefully you selected the appropriate wavelength and thought about your light intensity. If you ever considered adding dried flower petals, take a bow. But that’s exactly what Prof. Jan J. Weigand and coworkers at the Technische Universität Dresden did for several photoredox cross-coupling reactions (Ref 1). And to take a step back, it is a brilliant, incredibly fun well-characterized paper. One of our recent favorites.
As we all know by now, numerous photoredox synthetic methodologies have been developed for more than a decade (Ref 2). To many, light has the promise to be an efficient and clean energy source for synthetic chemistry. We certainly think so. But if you stop to think about your catalysts, you can quickly see the problems with basing so much of our chemistry on rare metals such as iridium or rhodium (or the synthetic requirements of the complex ligand systems). Metal-free synthetic dyes offer a solution but themselves require multistep syntheses and are often insufficient for the energy requirements of a reaction (more on this in a bit). So what are the opportunities for photoredox chemistry with organic dyes? Let’s jump in…
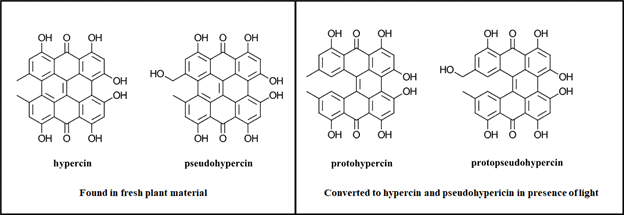
To find a sustainable catalyst, the authors looked to the plant genus Hypericum L. (hypericaceae) which includes 460 herbal species including Hypericum perforatum (St. John’s Wort). Hypericum perforatum is one of the world’s oldest medicinal herbs which some believe to be a cure everything from burns to depression or for use as an anti-viral compound (Ref 3). Certainly, someone out there has tested it against COVID-19 by now (Googling…oh, look they have and sadly it’s not the only hit?). Hypercin, one of the active ingredients in hypericum, is known to generate reactive oxygen species and believed to be responsible for hypericin’s phototoxicity towards bacteria and fungi. Additional, hypercin has been demonstrated to localize in cancer cells and has been studied for use in photodynamic therapy to kill cancer cells (Ref 4).
Figure 1: Hypercin analogues found in Hypericum
Relevant to this work, hypercin and pseudohypericin are found in fresh plant material with the highest levels of hypericin analogues found in the flowers. The unstable forms protohypericin and protopseudophypericin can be converted to hypericin and pseudohypericin with light (Figure 1). The authors selected ten species of hypericum (collected from Germany, Austria and Greece) for their initial screen. To prepare their catalysts, the plant material was dried for 24 h at 40 °C in the dark. The flowers were ground into a fine powder and added directly to the reaction mixture for the debromination cross coupling of 2-bromobenzonitrile and N-methyl pyrrole. (Figure 2)
Figure 2: Flower catalyzed chemistry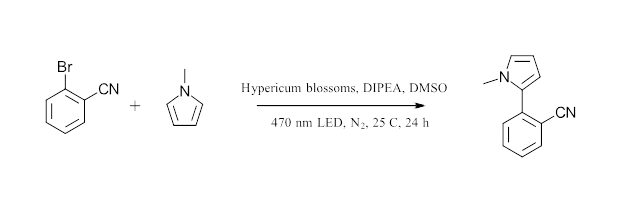
To everyone’s surprise (at least ours?), the reactions worked well with yields ranging from 31-68% varying by species of flower. The authors characterized the total concentration of the hypericin analogues found in each species by HPLC which varied greatly ranging from 0.016 wt% to 2.00 % (generally corresponding to conversion) as well as the concentration of individual analogues. For their controls, no reaction occurred in the absence of plant material or with plant material in the dark. For reactions performed with pure isolated hypericin analogues, each analogue demonstrated some activity with isolated hypericin having a similar conversion (73%) as the best reaction from the initial screen. With optimal conditions in hand, the authors expanded both the reaction scope and additional chemistry. Both hypericum vesiculosum flowers and isolated hypericin successfully catalyzed 30+ additional examples of “potpourri catalysis” (our term, not theirs). Importantly, the authors point out that the plant material can be removed with simple column chromatography. Check out the full paper for additional discussion on the reactivity of individual analogues, their prevalence in different species and an in-depth in situ UV-vis spectroelectrochemical study on the catalytic hypericin species and a complete mechanistic picture. Just a very nice work. Now, will we all be running our reactions tomorrow with flower blossoms? Probably not right away. But it is not unreasonable to think of a scenario where bulk plant matter could be useful on scale at some point.
Reusable Dyes
In the same light, with a focus on sustainability Pieber and coworkers from Max Planck recently reported a dye-based self-assembly system for nickel-catalyzed photoredox reactions (Ref 5). Numerous carbon-carbon and carbon-hetero bond formations using photoredox nickel catalysis have been reported with most utilizing iridium or ruthenium photocatalysts to initiate the cycle (Ref 6). Iridium and ruthenium bipyridine based photocatalysts are particularly useful due to their high excited state triplet energies and long-lived triplet state which can facilitate the bimolecular reaction with the Ni-catalyst. Many organic dyes have excited state redox potentials that theoretically are suitable to initiate the nickel catalysts (often with lower energy light); however, the short-lived singlet excited states of most dyes do not enable the reaction in homogenous solution. To overcome this problem, the authors report a series of dye-sensitized metallophotoredox catalysts (DSMP) containing an immobilized dye and nickel catalyst on a TiO2 bead (Figure 3).
Figure 3: Development of Dye-sensitized metallophotoredox catalysts (DSMP)
Happy to announce that our most recent paper came out in @ACSCatalysis today! Congratulation to first author @SusanneReisch for the excellent work @BioMolSys @MpiciPotsdam
https://t.co/SMo2mjhpNR— PieberLab (@PieberLab) November 2, 2020
Excitation of a series of dyes matched with the appropriate wavelength of light at 440, 525 and 666 nm afforded the excited photocatalysts which initiated electron transfer through the TiO2 and activating the nickel catalyst. The authors demonstrate a series of C-C, C-O, C-N and C-S couplings with the self-assembly catalysts that are recyclable (however with loss of yield in subsequent uses). A particular powerful demonstration of this technique of light and catalyst control is demonstrated in Figure 4. For the C-O arylation of (E)-cinnamic acid with iodobenzotrifluoride, the homogenous reaction with iridium and nickel catalyst gives a mixture of E:Z isomers with both blue and green light. Using the Fluoroscein-TiO2-Nidcbpy system DSMP system, the authors show that with green light, the E –isomer can be produced at 95% with the Z isomer not detected. While the extreme increase in reaction time (2 h to 72 h) can’t be overlooked neither can what can be presumed to be non-trivial purification necessary to separate the two isomers for this system or more complicated substrates. One can imagine many more uses for the DSMP technology and we look forward to what comes next.
Figure 4: Reaction selectivity of DSMP system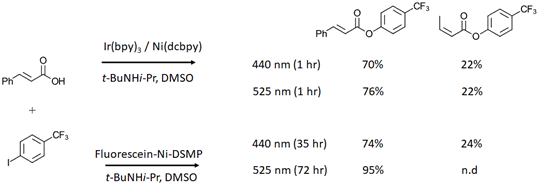
Finally, a quick note on two more metal-free photochemical reactions that we enjoyed recently (both open-access). First, Molander and coworkers report an inexpensive Hantzche ester based electron donor-acceptor complex for activating Ni(0) catalysts for sp3-sp2 (Ref 7). Second, a convenient way to access unnatural amino acids using mesityl-acrylate derivative photocatalysts by the Kärkäs group (Ref 8). The versatility of photoredox catalysis continues to expand with more and more useful examples, particularly as metal-free dyes become more prevalent.
Our most recent article on the implementation of photoactive electron-donor acceptor (EDA) complexes for Ni-mediated C(sp3)—C(sp2) bond formation is online ! Congrats to Lisa, Shorouk, and Ren-Ming !https://t.co/eqT0bogxEB pic.twitter.com/CQl1Deb7kh
— Molander Group (@molandergroup) March 6, 2021
References:
- Jun-jie Wang, Kai Schwedtmann, Kun Liu, Stephen Schulz, Jan Haberstroh, Gerrit Schaper, Anja Wenke, Julia Naumann, Torsten Wenke, Stefan Wanke and Jan J. Weigand, “Flowers of the plant genus Hypericum as versatile photoredox catalysts“ Green Chem. 2021, 23, 881.
- Peijun Li, Jack A. Terrett, and Jason R. Zbieg “Visible-Light Photocatalysis as an Enabling Technology for Drug Discovery: A Paradigm Shift for Chemical Reactivity”, ACS Med. Chem. Lett. 2020, 11, 11, 2120-2130.
- PatoÄka, “The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L.”, J. Appl. Biomed., 2003, 1, 61–70.
- Agostinis, A. Vantieghem, W. Merlevede and P. A. M. de Witte, “Hypericin in cancer treatment: more light on the way” Int. J. Biochem. Cell Biol., 2002, 34, 221–241.
- Susanne Reischauer, Volker Strauss, and Bartholomäus Pieber, “Modular, Self-Assembling Metallaphotocatalyst for Cross-Couplings Using the Full Visible-Light Spectrum”, ACS Catal. 2020, 10, 13269−13274.
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C. “The merger of transition metal and photocatalysis.” Rev. Chem. 2017, 1, 0052.
- Lisa Marie Kammer, Shorouk O. Badir, Ren-Ming Hu, and Gary A. Molander, “Photoactive Electron Donor-Acceptor Complex Platform for Ni-Mediated C(sp3)-C(sp2) Bond Formation” Chemical Science, 2021, ASAP.
- Andrey Shatskiy, Anton Axelsson, Elena V. Stepanova, Jian-Quan Liu, Azamat Z. Temerdashev, Bhushan P. Kore, Björn Blomkvist, James M. Gardner, Peter Dinér, and Markus D. Kärkäs, “Stereoselective Synthesis of Unnatural α-Amino Acid Derivatives through Photoredox Catalysis” Chemical Science, 2021, ASAP.