Built-in efficiencies make Hepatochem’s one of the best photoredox flow reactors available.

The common limitation to scaling up photoredox chemistry is due to the low penetration of the light in to the reaction mixture (few mm) which prohibits the use of large reaction vessels. Surface area is key to shorten reaction time. It is possible to significantly increase the surface area by running the reaction in flow. This will decreases the reaction time and allows to be run in continuous mode for scale-up.
To solve this challenge, we designed a flow reactor that can be used in the PhotoRedOx Box™. This flow reactor is using PFA tubing and has volume of 2 ml. Comparing reactions in flow and in batch we observed significant decrease in reaction time.

Photoredox Flow Reactor
HCK1006-01-022
(Patent Pending)
Photoredox Flow Reactor Validation Reaction 1
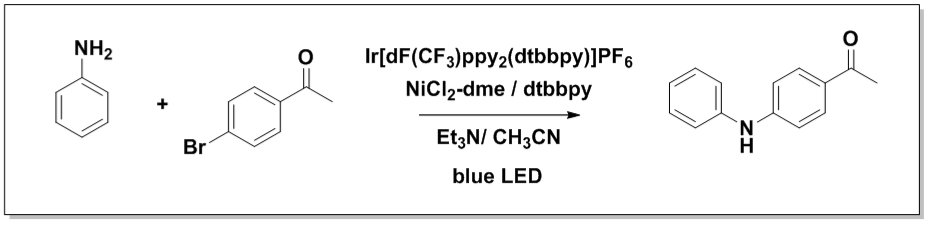
Time to 95% conversion: Flow 30 min, batch 24h
Reaction Protocol:
In a 4-ml vial equipped with a Teflon septa were weighed NiCl2-dme (1.1 mg, 5 μmol, 5 mol %) and dtbbpy (1.3 mg, 5 μmol, 5 mol %). 1 ml of dry MeOH was added to the vial and the vial was stirred on an orbital shaker until complete dissolution. The solution was evaporated to dry at room temperature. Then Ir(dF-CF3-ppy)2(dtbpy) (1.1 mg, 1 μmol, 1 mol %), and 4-bromoacetophenone (9.95 mg, 100 μmol, 1 equiv.) were added. 1 ml of dry acetonitrile was added followed by Et3N (21 μmol, 300 μmol, 3 equiv.) and aniline (4.65 mg, 100 μmol, 1 equiv.). The solution was sparged with nitrogen via submerged needle for 5 minutes.
Several batches of 100 μl of solution were successively injected to the flow reactor placed in EvoluChem PhotoRedOx Box with blue Kessil LED using an injection module (Gilson) and the samples were circulated using a HLPC pump at different flow rates to allow residence time of 5, 10, 15, 20 and 30 min. Reaction completion was monitored by LC-MS using the ratio bromoacetophenone/product.
Flow Reactor Validation Reaction 2
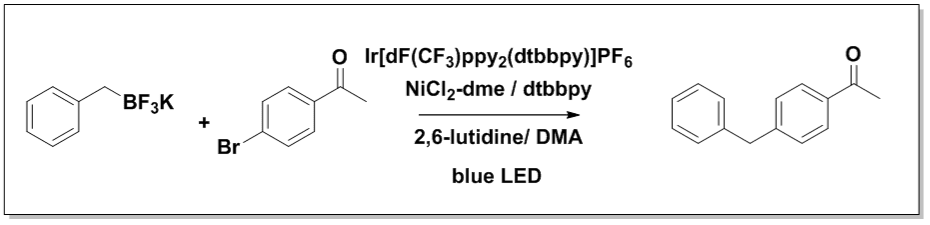
Time to 95% conversion: Flow 30 min, batch 8h
Reaction Protocol:
In a 4-ml vial equipped with a Teflon septa were weighed NiCl2-dme (1.1 mg, 5 μmol, 0.1 mol %) and dtbbpy (1.3 mg, 5 μmol, 0.1 mol %). 1 ml of dry MeOH was added to the vial and the vial was stirred on an orbital shaker until complete dissolution. The solution was evaporated to dry at room temperature. Then Ir(dF-CF3-ppy)2(dtbpy) (1.1 mg, 1 μmol, 0.1 mol %), and 4-bromoacetophenone (4.98 mg, 50 μmol, 1 equiv.) were added. 1 ml of dry acetonitrile was added followed by 2,6 lutidine (17.5 μmol, 150 μmol, 3 equiv.) and potassium benzyltrifluoroborate (9.90 mg, 50 μmol, 1 equiv.). The solution was sparged with nitrogen via submerged needle for 5 minutes.
Several batches of 100 μl of solution were successively injected to the flow reactor placed in EvoluChem PhotoRedOx Box™ with blue Kessil LED using an injection module (Gilson) and the samples were circulated using a HLPC pump to allow residence time of 30 min. Reaction completion was monitored by LC-MS using the ratio bromoacetophenone/product.

