
For this month’s newsletter we thought we would look at an unexpected surprise in photochemistry. A project with one of those moments where “hey that’s weird, and very wrong” turns into something bigger and better. It takes a bold decision, a little stubbornness and some ingenuity, to turn a potential dead end into a new opportunity.
This brings us to a recent paper by the A. Stephen K. Hashmi and coworkers in Nature Communications titled “An unexpected synthesis of azepinone derivatives through a metal-free photochemical cascade reaction” (Open Access) (Ref 1). “Unexpected synthesis” is not a topic that shows up all that often in the literature. Synthesis is planned, meticulously so. Unexpected synthesis is usually the black tar on the bottom of your flask that you try to scrape out with a spatula before throwing the whole works in the trash. And when “unexpected” happens to give you something useful, by the time you get around to writing up the paper usually there is a well-reasoned explanation for why you planned it in the first place. So, it’s refreshing to see all of that stated up front.
The authors were attempting to look at the reactivity of 2-aryloxyaryl nitrenes. This was based on their previous work using sulfilimines as nitrene precursors via both photocatalysis and Rh catalysis to generate carbazoles (Figure 1A). For this work, the expectation was that they would form the 10H-phenoxazine derivatives upon irradiation of the sulfilimine via a nitrene intermediate. Instead, they formed an azepinone, which in case you aren’t up on your heterocycle nomenclature is the oxidized form of an azepine which is in turn a 7-membered unsaturated heterocycle containing 1 nitrogen (Figure 1B). Side note: 7 membered rings will always look goofy to us. If you can draw one freehand with a regular shape you are better than us. It always just looks like an accidental mishappened cyclohexane or 1 carbon short of an octane. It just never looks right. Maybe that’s just us.
Figure 1: Unexpected azepinone formation (Figure adapted from Ref 1)
A. Previous Work
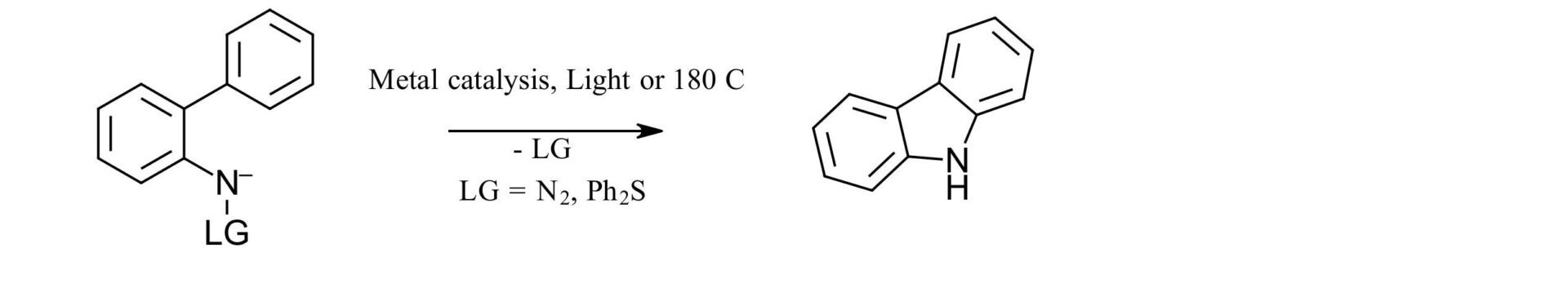
B. This Work
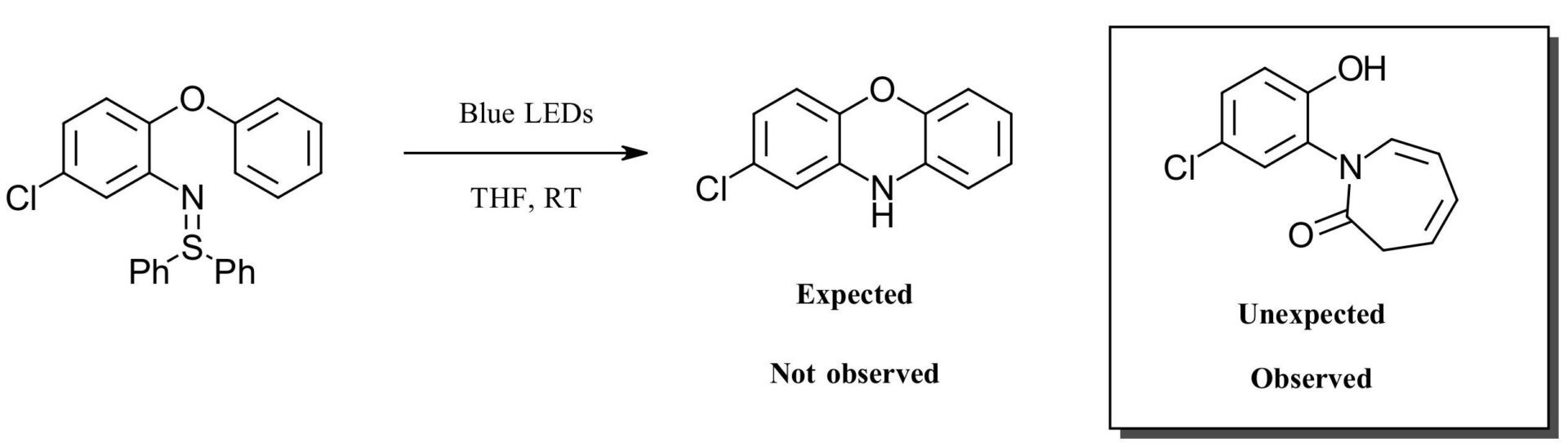
Azepinones are found in many natural products and pharmaceuticals as privileged structures. Methods to make these compounds usually require a strong base, high power UV light or metal catalysis. A metal free visible light catalysis method for making a series azepinones inexpensively? Yeah, that could work.
But first, how did they get here? And can they do it again?
Optimization of the method found a few key features. First, blue light (40% conversion) performed better than UVA (17%). Switching to solvents other than THF was detrimental. Using the azide in place of the sulfilimine and adding 10 equiv. of H2O pushed the yield to 65%. And finally, 0.5 equiv. p-toluenesulfonic acid was best at 83% for this model system. Other protic acids and Lewis acids showed no improvement. And ultimately, no reaction was observed in the dark.
Figure 2: Model reaction for generation of a series of azepinone derivatives
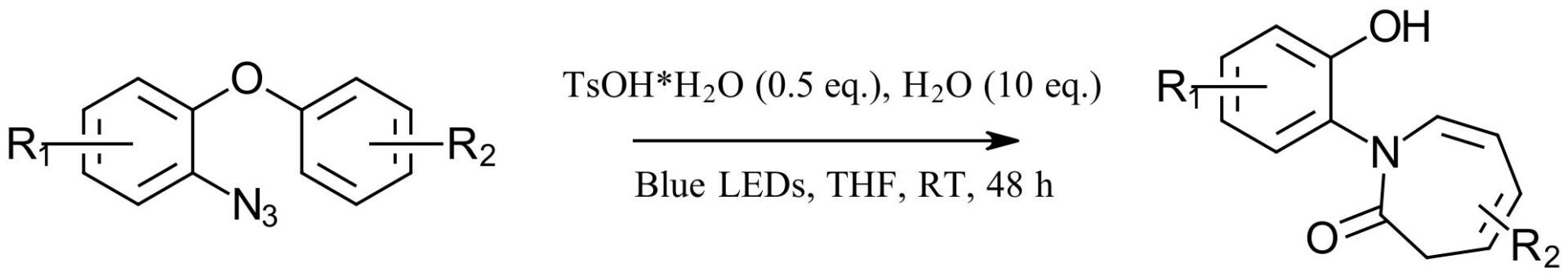
With reaction in hand, the authors then looked at an extensive substrate scope with several of the resulting azepinone structures further derivatized to more complex structures. The aryl azide (R1) supported chloro, trifluormethyl, methyl and methoxy groups and the arene (R2) supported alkyl and arene and thiophenyl substituents. The phenol can be easily protected and/or converted with additional coupling reactions for more complex products. Ultimately, this method looks to be an inexpensive method for generating a library of azepinone derivatives.
For the mechanism? Reactions with Da‚‚O gave 50% deuterium incorporation into the CHa‚‚ on the azepinone and 95% in the phenol. 18O-labeling experiments with Ha‚‚18O gave 90% 18O incorporation into the carbonyl. Ultimately, DFT and the labeling experiments support a mechanism proposing the following cascade reaction, photochemical generation of a 2-aryloxyaryl nitrene, [2+1] annulation, ring expansion, and addition of water.
Figure 3: Selected steps in the proposed cascade reaction
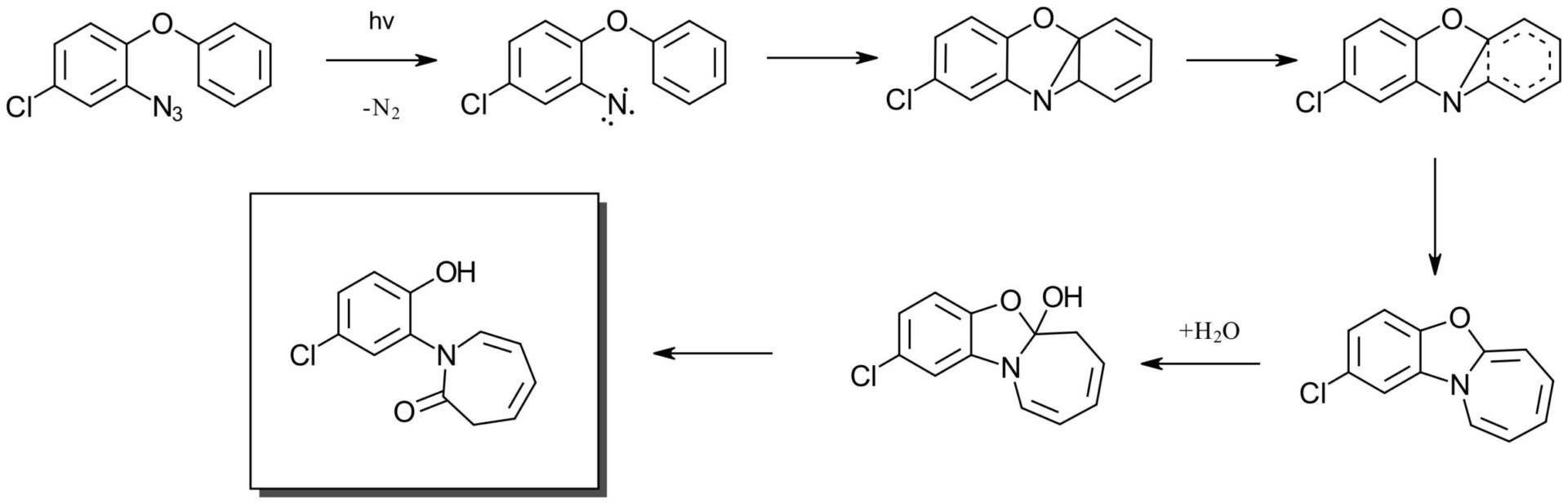
In the end, this unexpected result that wasn’t discarded or ignored ended up resulting in an inexpensive versatile reaction suitable for a generating libraries of an important. Great work!
References:
Song, L.; Tian, X.; Farshadfar, K.; Shiri, F.; Rominger, F.; Ariafard, A.; Hashmi, A. S. K. An Unexpected Synthesis of Azepinone Derivatives through a Metal-Free Photochemical Cascade Reaction. Nat. Commun. 2023, 14 (1), 831. https://doi.org/10.1038/s41467-023-36190-z.

