 A recurring theme for many of our articles over the last few months is that there just isn’t enough iridium or ruthenium in the earth’s crust to do all of the photochemistry that we would like to perform at scale.
A recurring theme for many of our articles over the last few months is that there just isn’t enough iridium or ruthenium in the earth’s crust to do all of the photochemistry that we would like to perform at scale.
Due to cost and the scarcity of these metals, the development of organic dyes such as benzo[ghi]perylene imides (Ref 1) or acridinium salts (Ref 2) among many others are increasingly common (Also, flower petals!). But if we are sticking with metals, it would be great if we had access to sustainable alternatives like the use of earth abundant transition metals complexes. There is an extensive precedent swapping in iron, copper or nickel for traditional organometallic chemistry that previously required palladium, platinum, rhodium, etc. However, while swapping in an abundant earth metal complex for photocatalysis would also be quite desirable, is it far less common and potentially more challenging.
The use of Ir and Ru photocatalysts is so prevalent, despite their cost and scarcity, because they are fairly perfectly suited for photoredox catalysis. Ir and Ru afford long excited state lifetimes (microseconds), strong absorbance in the visible region, stable metal-ligand bonds, and high excited reduction and oxidation potentials to readily undergo single-electron transfer (SET) or energy transfer (EnT). In comparison abundant earth metals often have ultrashort excited lifetimes (pico to nanosecond), labile metal-ligand bonds and less favorable oxidant potentials. All of which makes bimolecular processes to harvest photochemical energy with 3d transition metals difficult. But hey, iron is cheap. Really, really cheap. And so, with that driving force, is an opportunity to think about different reaction mechanisms and paradigms to take advantage of photochemistry without precious metals.
In a recent review in Angewandte Chemie, Oliver Reiser and coworkers discuss the photochemistry of earth-abundant metals in “Visible-Light-Induced Homolysis of Earth-Abundant Metal-Substrate Complexes: A Complementary Activation Strategy in Photoredox Catalysis” (Ref 3). The authors are focusing on examples of visible-light induced homolysis (VLIH) of organometallic complexes, so the few examples of abundant earth metals acting as traditional photocatalysts or co-catalysts are not discussed in detail. Instead, the authors focus on examples of using pre-functionalized organometallic complexes of 3d transition metal complexes that can take advantage of the photo-labile metal-ligand bonds.
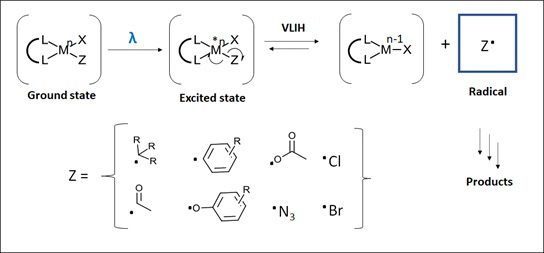
An overarching general scheme for the VLIH strategy involves first forming a ground state metal-substrate complex [LnMn(X)-Z] (Figure 1). This can be achieved through many ways that are familiar in organometallic chemistry such as ligand exchange, oxidative addition, single-electron addition or transmetallation. Excitation of the metal-substrate complex with visible light affords a generally short-lived excited [LnMn(X)-Z]* species. Homolysis of the M-Z bond through various inner sphere redox processes affords the substrate radical Z· ready for further modification. The paper then proceeds to describe examples of the different mechanism aspects of the M-Z bond homolysis and the implications for the desired chemistry based on 3d transition metal complexes including copper, iron, nickel, cobalt, cerium and vanadium.
Figure 1: General scheme for visible-light induced homolysis (VLIH). Adapted from Ref 3 – Figure 1
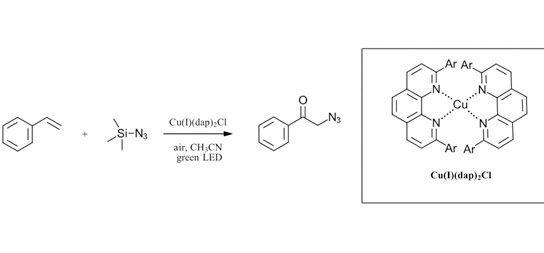
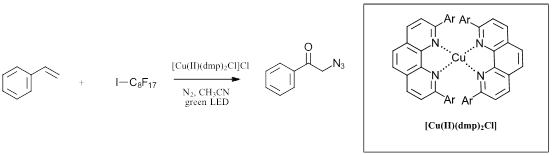
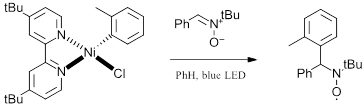
For copper, several interesting examples have recently been described for violet, blue, green and white LEDs. As shown in Figure 2, Reiser and coworkers recently have described several copper catalyzed functionalization of olefins (Ref 4, 5). Nickel complexes have shown perhaps the most promised for photocatalysis both in conjunction with iridium catalysts for cross-coupling reactions with blue LEDs (Ref 6) and on their own at lower wavelengths (Ref 7). As an example of a nickel complex through a VLIH mechanism, the review discusses the recent report by Doyle and coworkers thoroughly investigating the homolysis of Ni intermediates Figure 3 (Ref 8). Take a look at the full review for in depth discussion of the mechanistic implications of the VLIH mechanism for abundant-earth metals with examples for a variety of 3d metals.
Figure 2: Recent examples of copper complexes for VLIH reactions (Ref 4, 5)
Figure 3: VLIH of a Ni(II) species (Ref 8)
Since this review was published, copper shows up again in photoredox catalysis in a big way, in this recent paper in Chem by MacMillan and coworkers using a copper system for alkylation of a wide range of substrates (Ref 9). While also using iridium, this paper represents the increasing number mechanistic possibilities combining catalysis and light. We are certainly going to see many more examples of 3d transition metals involved in photocatalysis in the future.
In their latest work – now online at Chem – @MacMillan_Lab describe a general, room temperature N-alkylation via copper metallaphotoredox catalysis. Read more here: https://t.co/AoplZgG0y3
– Chem (@Chem_CP) June 17, 2021
References:
- MacKenzie, I. A.; Wang, L.; Onuska, N. P. R.; Williams, O. F.; Begam, K.; Moran, A. M.; Dunietz, B. D.; Nicewicz, D. A. Discovery and Characterization of an Acridine Radical Photoreductant. Nature 2020, 580 (7801), 76–80. https://doi.org/10.1038/s41586-020-2131-1.
- Cole, A. J. P.; Chen, D.; Kudisch, M.; Pearson, R. M.; Miyake, G. M. Organocatalyzed Birch Reduction Driven by Visible Light. Am. Chem. Soc 2020, 142 (31), 13573–13581. https://dx.doi.org/10.1021/jacs.0c05899
- Abderrazak, Y.; Bhattacharyya, A.; Reiser, O. Visible”Light”Induced Homolysis of Earth”Abundant Metal”Substrate Complexes: A Complementary Activation Strategy in Photoredox Catalysis. Chemie Int. Ed. 2021, Early view. https://doi.org/10.1002/anie.202100270.
- Hossain, A. Vidyasagar, C. Eichinger, C. Lankes, J. Phan, J. Rehbein, O. Reiser, Angew. Chem. Int. Ed. 2018, 57, 8288 – 8292; Angew. Chem. 2018, 130, 8420 – 8424.
- Engl, O. Reiser, Eur. J. Org. Chem. 2020, 1523 – 1533.
- Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. Photoredox Catalysis in Organic Chemistry. Org. Chem. 2016, 81 (16), 6898–6926. https://doi.org/10.1021/acs.joc.6b01449.
- Lim, C. H.; Kudisch, M.; Liu, B.; Miyake, G. M. C-N Cross-Coupling via Photoexcitation of Nickel-Amine Com-Plexes. Am. Chem. Soc. 2018. https://doi.org/10.1021/jacs.8b03744.
- I. Ting, S. Garakyaraghi, C. M. Taliaferro, B. J. Shields, G. D. Scholes, F. N. Castellano, A. G. Doyle, J. Am. Chem. Soc. 2020, 142, 5800 – 5810
- Dow, N., Cabre, A. and MacMillan D. W. C. , A general N-alkylation platform via copper metallaphotoredox and silyl radical activation of alkyl halides, Chem (2021), https://doi.org/10.1016/j.chempr.2021.05.005