Setting Up Your Initial Photochemistry Reactions
This is the third and final part of a three part series designed to help you get started by understanding light sources in photochemistry. Missed the start of the series where we cover the basics and core principles? No worries, you can read it here…
Visible light photoredox catalysis uses the excited states of metal complexes and organic dyes to perform energy transfer and single-electron transfer (SET) processes for an ever-increasing number of useful synthetic transformations. As should be apparent from reading Part 1 (/photochemistry-101-everything-you-need-to-know-to-get-started-part-i/) and Part 2 (/photochemistry-101-part-ii-understanding-and-measuring-light-sources/), we think photochemistry is pretty cool. But is it useful? The possibilities of where to start first can seem daunting. A standard reaction setup is imperative for reproducible chemistry from lab to lab enabling a low barrier to entry for the field. As you just read, step one to standardizing reactions is a better understanding of the light source. The second step is the reactor. Let’s do a quick review before we start providing details on your initial photochemistry reactions.
First, it is a simple concept at first but needs to be stated. The only light that is useful to running your reaction is the light that actually makes its way into the flask. The bright blue light shining on the back of your hood isn’t doing anything. For this reason, we have developed a series of photoreactors to maximize light intensity, control temperature and standardize reaction conditions that are currently in use in both industrial and academic settings (Ref 20). For use with a standardized setup, (the EvoluChem Photoredox Box), we have selected and adapted four reactions from the literature as a convenient starting point for those new to photochemistry. Each reaction has been tested and validated in our equipment. Each is available as a part of bundle including photoreactor, sample holders, LED’s and premixed reactions to run test reaction, as well as three substrate combinations of your choice (see https://hepatochem.com/photoreactors-leds-accessories/photochemistry-starter-bundle/).
Experimental Details
Each reaction is performed in the Evoluchem PhotoRedOx box, equipped with either an 18W 450 nm or 365 nm Evoluchem LED. Reactions are performed in 4 mL vials equipped with a Teflon septa cap containing pre-weighed photocatalyst, co-catalyst base and reagents. Substrate solutions are added via syringe and the reaction is sparged with a N2 line via needle for 5 minutes prior to turning on the LED’s. The reactor is equipped with a fan that holds the reaction temperature stable at ~30 °C. Reactions are run for 18-24 hr. Product analysis is performed by LC-MS.
Initial Photochemistry Reactions:C-C cross-coupling with amino acid decarboxylation
Adapted from Ref 14
The first reaction that we want to highlight is an Iridium/nickel catalyzed carbon-carbon bond formation as described by MacMillan and coworkers (Ref 14). This approach uses a commercially available iridium catalyst (Ir[dF(CF3)ppy]2(dtbbpy)PF6 (structure in Figure 2) with a CFL bulb. The photoredox cycle activates a nickel catalyzed organometallic cycle for the coupling of α-carboxyl sp3-carbons with aryl halides. The nickel catalyst is formed in situ between NiCl2 with dtbbpy as a ligand. The reaction requires a base (Cs2CO3) and sparging with nitrogen to remove oxygen. The reaction demonstrated coupling for a wide range of carboxylic acids with aryl bromides, iodides and select chlorides. The test reaction we have selected is the coupling of N-Boc-Valine and 4-bromoacetophenone which we have adapted from the reported procedure to use 450 nm LED instead of the CFL (Figure 8).
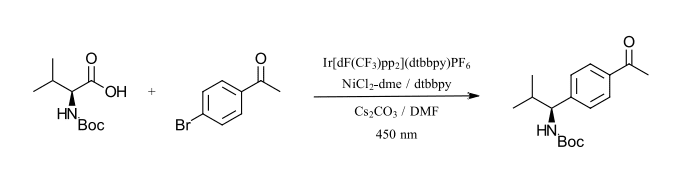
Figure 8: Carbon-carbon formation between sp3 carbons and aryl halides (adapted from Ref 14)
Initial Photochemistry Reactions:C-O bond formation
The second reaction that we have selected is also by MacMillan and coworkers using Ir/Ni catalysis, however this time for C-O bond formation (Ref 21). This reaction also uses (Ir[dF(CF3)ppy]2(dtbbpy)PF6, NiCl2 and dtbbpy as a ligand. This reaction requires quinuclidine as an electron donor/acceptor and an additional base. The reaction uses primary and secondary alcohol for C-O bond formation with aryl bromides. The reaction requires a base (K2CO3) and sparging with nitrogen to remove oxygen. Nickel itself is unable to perform C-O couplings without the use of high temperatures due to the stability of nickel-alkoxide complexes. The photoredox cycle using a 450 nm LED and the iridium catalyst can activate this cycle at room temperature for a wide variety of alcohols and aryl bromides. The test reaction that we have selected is the coupling of cyclohexanol and 4-bromoacetophenone with 450 nm (Figure 9).
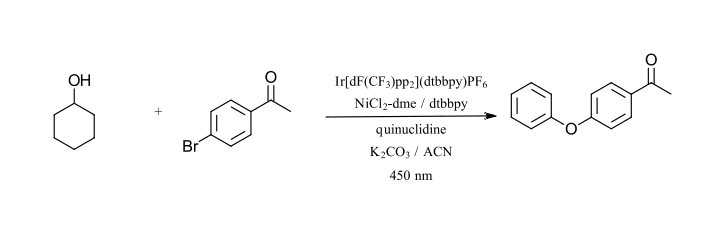
Figure 9: C-O bond formation (Adapted from Ref 21)
Initial Photochemistry Reactions:C-C Cross coupling with BF3K reagents
The third reaction that we want to highlight is also a C-C bond formation, however this time using an alkyl BF3K reagent as the coupling partner. Molander and coworkers have performed a substantial amount of work using BF3K reagents for photoredox catalyzed transformations. The reaction we are focusing on is the Iridium/nickel catalyzed cross coupling of aryl bromides with secondary alkyl BF3Kreagents (Ref 22). BF3K reagents are easy to handle, bench stable solid reagents useful for many cross-coupling reactions. This reaction works extremely well for a wide range of alkyl-BF3Kwith aryl bromides and can very quickly be used to generate a large series of analogues. The reaction requires a base (Cs2CO3) and sparging with nitrogen to remove oxygen. The test reaction we have selected uses cyclohexyl-BF3K and 4-bromoacetophenone (Figure 10). We have modified the reaction condition to use Ir[dF(CF3)ppy]2(dtbbpy)PF6 instead of Ir[dF(CF3)ppy]2(bpy)PF6(slightly different ligand), lowered the catalyst loading and use 450 nm LEDs instead of CFL.
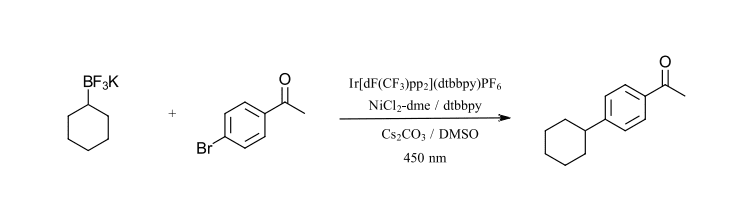
Figure 10: C-C cross-coupling with BF3K reagents (adapted from Ref 22)
Initial Photochemistry Reactions:C−N Cross-Coupling via Photoexcitation of Nickel−Amine Complexes
The final reaction we have selected is a carbon-nitrogen cross coupling reaction as described by Miyake and coworkers (Ref 23). This is the first reaction that we have selected that does not include an iridium catalyst or 450 nm LED. Here, the inexpensive NiBr2 salt forms a photoactive complex with primary and secondary amines that can be excited by 365 nm LED’s to give C-N bond formation products using aryl bromides. When excess amine is used, the addition of quinuclidine is not required. The reaction should be sparged with nitrogen to remove oxygen. Of note, this reaction is run at higher concentration than the previously selected reactions and the Ni-amine complex could be described as the photocatalyst for the reaction. We have selected the coupling of morpholine with 4-bromoacetophenone without quinuclidine.
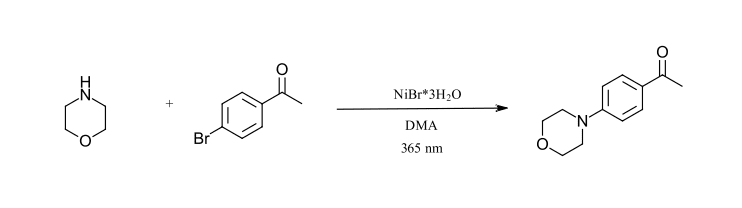
Figure 11: C-N Cross-coupling reaction (Ref 23)
We hope you have enjoyed this series on Photochemistry 101 and invite you to email us (info@hepatochem.com) or follow us on Twitter (@EvoluChem) to suggest more content and subject areas you would like us to cover. If you have any questions about the experiments above, or just find yourself stuck and looking for a good listener… drop us a line! Check out our starter bundle and give photoredox catalysis a try!
You just read the third and final part of a three part series designed to help you get started in photochemistry. Below are links to all three parts of the series. Any questions? Send them to info@hepatochem.com, we’d love to hear from you!
Here’s the entire series:
Photochemistry 101, Part I: Everything You Need To Know To Get Started
Photochemistry 101, Part II: Understanding and Measuring Light Sources
Photochemistry 101, Part III: Setting Up Your Initial Photochemistry Reactions
References
- Yes, this is a simplified explanation, there are entire textbooks written about this stuff.
- https://hepatochem.com/red-light-applications-in-photochemistry/
- Don’t worry, there’s still room for you to synthesize 50 nearly identical derivatives of your favorite chromophore.
- https://hepatochem.com/electron-donor-acceptor-eda-complexes-in-photochemistry/
- Tucker, J. and Stephenson, C. R. J. “Shining Light on Photoredox Catalysis: Theory and Synthetic Applications”, Journal of Organic Chemistry, 2012, 77, 1617-1622.
- Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886.
- Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756.
- Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
- Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898−6926. https://pubs.acs.org/doi/abs/10.1021/acs.joc.6b01449
- Romero, N., Nicewicz, “Organic Photoredox Catalysis”, Chemical Reviews, 2016 (116), 10075-10166.
- Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Visible light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem., Int. Ed. 2018, 57, 10034−10072.
- Harper, K. Moschetta, E., Bordawekar, S., Wittenberger, S. “A Laser Driven Flow Chemistry Platform for Scaling Photochemical Reactions with Visible light., ACS Central Science, 2019 (5), 109-115.
- Justin P. Cole, Dian-Feng Chen, Max Kudisch, Ryan M. Pearson, Chern-Hooi Lim, and Garret M. Miyake, “Organocatalyzed Birch Reduction Driven by Visible light, J. Am. Chem. Soc, 2020, 142, 13573-13581. https://pubs.acs.org/doi/abs/10.1021/jacs.0c05899
- Zuo, Z., Ahneman, D., Chu, L., Terrett, J., Doyle, A., Macmillan, D. “Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides” Science, 2014 (345), 437-440.
- Bonfield, H.E., Knauber, T., Lévesque, F. et al. Photons as a 21st century reagent. Nat Commun 11, 804 (2020) https://doi.org/10.1038/s41467-019-13988-4
- https://hepatochem.com/evaluating-light-sources-in-photochemistry/
- https://hepatochem.com/determine-photon-flux-using-actinometry/
- Hatchard C.G.; Parker C.A. “A new sensitive chemical actinometer. 2. Potassium ferrioxalate as a standard chemical actinometer.” Proc. R. Soc. London, Ser. A. 1956, 235, 518-536.
- https://hepatochem.com/standard-ferrioxalate-actinometer-protocol/
- https://hepatochem.com/photoreactors-leds-accessories/#photoreactors
- Terret, J. Cuthbertson, J. Shurtleff, V. MacMillan, D. “Switching on elusive organometallic mechanisms with photoredox catalysis”. Nature, 2015, 524, 330-334.
- Primer, D., Karakaya, I. Tellis, J. Molander, G. “Single-Electron Transmetallation: An Enabling Technology for Secondary Alkylboron Cross-Coupling”. J. Am.Chem. Soc. 2015, 137, 2195.
- Lim, C.H., Kudisch, M., Liu, B., Miyake, G. “C-N Cross-Coupling via Photoexcitation of Nickel-Amine Complexes” J. Am. Chem. Soc. 2018, 140, 24, 7667-7673.

