Abtract
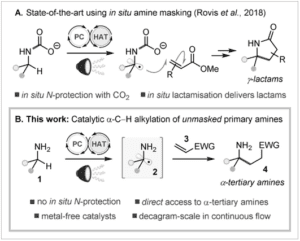
A)”…Prior art for catalytic α-C-H alkylation of primary amines; B)”…This work. EWG=electron”withdrawing group.
A practical, catalytic entry to α,α,α”trisubstituted (α”tertiary) primary amines by C-H functionalisation has long been recognised as a critical gap in the synthetic toolbox. We report a simple and scalable solution to this problem that does not require any in situ protection of the amino group and proceeds with 100″‰% atom”economy. Our strategy, which uses an organic photocatalyst in combination with azide ion as a hydrogen atom transfer (HAT) catalyst, provides a direct synthesis of α”tertiary amines, or their corresponding γ-lactams. We anticipate that this methodology will inspire new retrosynthetic disconnections for substituted amine derivatives in organic synthesis, and particularly for challenging α”tertiary primary amines.
Authors: Alison S. H. RyderDr. William B. CunninghamGeorge BallantyneTom MulesAnna G. KinsellaJacob Turner”DoreDr. Catherine M. AlderLee J. EdwardsDr. Blandine S. J. McKayDr. Matthew N. GraysonDr. Alexander J. Cresswell
Link
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202005294#.Xr2aciKIkvU.twitter

