Flow photochemistry in the PhotoRedOx Box™ is Hot as F!
When we see an EvoluChem PhotoRedOx Box™ used in creative ways, we feel obliged to share it as loudly as possible. Recently, we wrote about photocatalytic methods for Hot labels using HAT for deuterium and tritium labeling that used the PhotoRedox Box TC™. This month, we have this amazing work from Veronique Gouverneur and coworkers in JACS entitled “Photoredox Nucleophilic (Radio)fluorination of Alkoxyamines” (Ref 1) developing a novel photoredox induced fluorination for radiolabeling a wide range of pharmaceutically relevant small molecules. The optimized reaction was then applied to a radiosynthesizer which incorporated a PhotoRedOx Box Flow Reactor™ and blue LED as part of a flow process ultimately affording an automated radiosynthetic platform with fast access to 18F labeled aliphatic fluorides. An amazing work—but we’re getting ahead of ourselves.
Why would someone make so much effort to incorporate radioactive 18F into a small molecule? The answer is Positron Emission Tomography (PET). PET is a sensitive non-invasive imaging technique for real time monitoring in vivo with numerous diagnostics uses for detecting cancers, metabolic conditions, organ failure, blood flow and on and on. Because of both its decay profile (97% β+) and half-life of 109.7 min of 18F, radiolabeled small molecules containing F are a great source of positrons for imaging. As many drugs already contain a 19F, radiolabeled derivatives can be utilized in the identical manner of the unlabeled drug, although synthetic methods for incorporating the radiolabeled 18F can be limiting. A good synthetic method for radiolabeling with 18F requires a few things. An efficient mild reaction, performed quickly, with a simple purification. And the ability to be performed onsite or near an imagining site so that the labeled compound can be utilized efficiently.
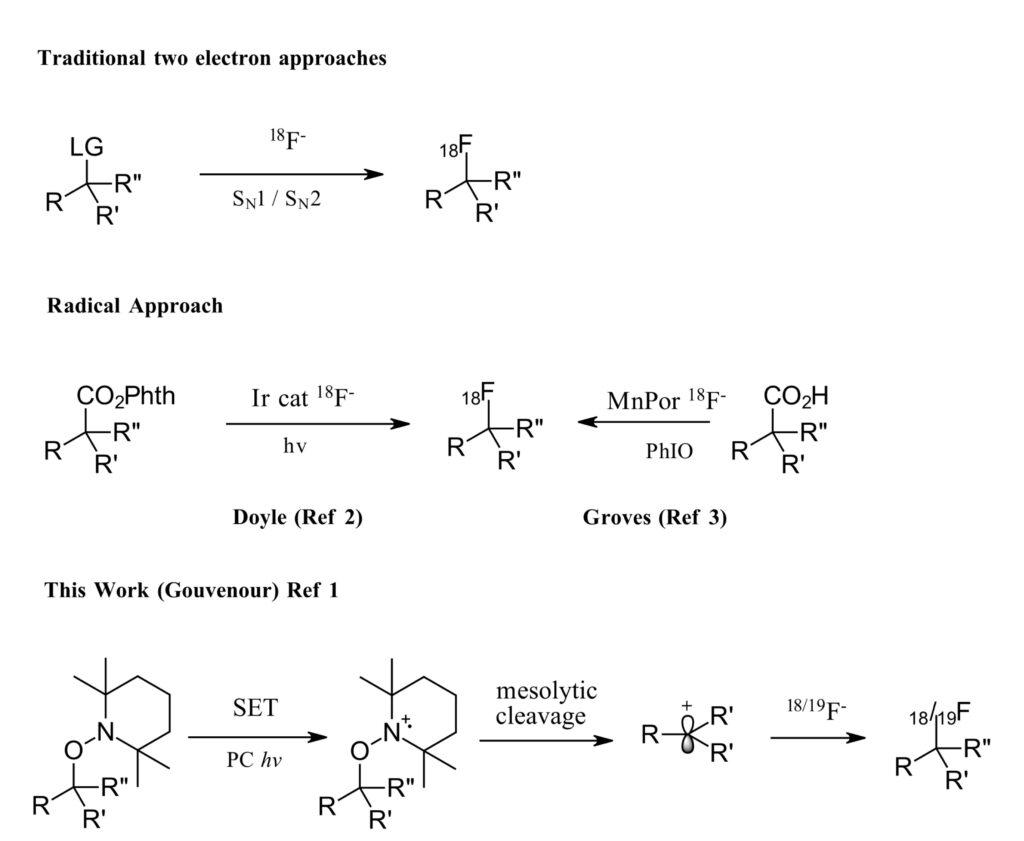
In recent years, methods for two-electron pathways to fluorination of small molecules are quite abundant (Figure 1). However, most require complex synthesis of pre-functionalized precursors as an efficient leaving group, harsh conditions and have poor reactivity for secondary or tertiary substrates. Two recent examples of radical fluorination are also described, with limitations on accessible substrates, selectivity and purification issues (Ref 2,3). When you start to think about the requirements for synthesis on demand, with limitations on availability of labeled fluorine sources, short time frames and purification complications, the requirements for a successful radiolabeling fluorination reaction become apparent. As such the author’s stated goal is as follows, “to offer radiochemists a novel versatile method to prepare alkyl 18F-fluorides using a broader range of both starting materials and carbocations”.
Figure 1: Methods for Fluorination (adapted from Ref 1-Figure1)
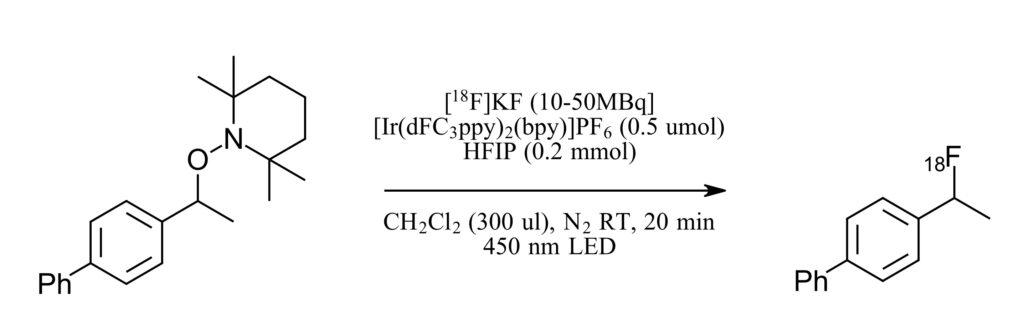
The authors selected TEMPO-derived alkoxyamines, which are suitable for photoredox induced functionalization, although fluorination with them has not been previously described. TEMPO-derived substrates are easily accessible in a single step from a diverse set of functional handles including carboxylic acids, halides, alkenes, alcohols, aldehydes, boronates and C-H bonds. For fluorine sources, the authors were only interested in fluorine reagents that would be suitable for radiolabeling and so common reagents such as SelectFluor (requiring F2 for synthesis, not practical at a radiolabeling facility) or NEt3·3HF (successful initial reaction of 84% but not compatible for automation) were not thoroughly investigated. Instead, the authors focused on KF and CsF two fluorine sources accessible as 18F that are easily handled. And so, the authors set about extensively optimizing their reaction with an eye towards every detail mattering for automation. HFIP was added as a proton source and solubilizing agent. The reaction proceeded well with low substrate loading. An ionic iridium photocatalysts was selected over other options, ultimately for suitability in purification from automated reaction by a cartridge. Also, the reaction proceeded without need for degassing with nitrogen. Ultimately a reaction was discovered that proceeds quickly, with 71% radiochemical yield in 2 minutes of irradiation time (92% at 20 minutes).
Figure 2: Model reaction for optimization
The authors then screened a broad selection of substrates containing a wide array of functional groups found in medicinal chemistry, with each TEMPO-derived version of each accessible in one step. Most of the reactions proceeded with greater than 70% conversion for labeling with 18F. (Check out the paper to see the full substrate scope). In addition, the reaction proceeding with unpurified TEMPO-derived substrates without loss of reactivity or labeling demonstrates a one pot method for access to radiolabeled 18F compounds.
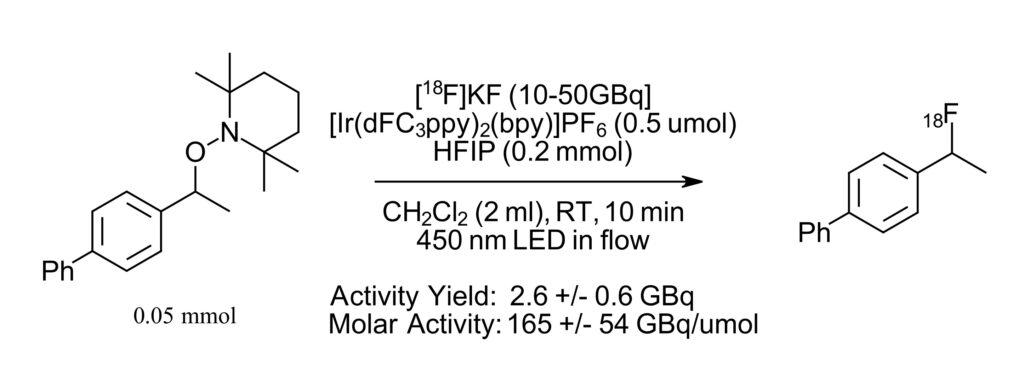
Figure 3: Reaction in radio-synthesizer with PhotoRedox Box™
With the reaction in hand, the authors then set out to perform the photocatalytic radiofluorination reaction in an automated system. Here the PhotoRedOx Box Flow Reactor™ was utilized with a blue LED because it was easily incorporated in flow into a TRASIS AllinOne radio-synthesizer. The number of steps and reaction details that can be automated in the radio-synthesizer is quite impressive and we really can’t do it justice in this short summary. (but check the Supporting information of the paper if you are interested). Notably, all the of the steps required are incorporated. From the loading of each reagent, drying of the 18F labeled fluoride source, mixing of reagents, transfer to the PhotoRedOx Box Flow Reactor™ with 450 nm LED, back into the synthesizer for purification on a C18 cartridge followed by analysis by radio-HPLC with the entire process taking less than 90 minutes.
In the end, the authors successfully developed “the first redox-neutral, light-mediated nucleophilic fluorination of alkoxyamines, and demonstrate suitability for 18F-labeling and applications in PET imaging.” The automated process takes advantage of two commercial instruments with easy operation and should be broadly applicable to other flow photoredox processes. Great work!
References
- Ortalli, S.; Ford, J.; Trabanco, A. A.; Tredwell, M.; Gouverneur, V. Photoredox Nucleophilic ( Radio ) Fluorination of Alkoxyamines. J. Am. Chem. Soc 2024, 0–5. https://doi.org/10.1021/jacs.4c02474.
- Webb, E. W.; Park, J. B.; Cole, E. L.; Donnelly, D. J.; Bonacorsi, S. J.; Ewing, W. R.; Doyle, A. G. Nucleophilic (Radio)Fluorination of Redox-Active Esters via Radical-Polar Cross- over Enabled by Photoredox Catalysis. J. Am. Chem. Soc. 2020, 142, 9493−9500. https://pubs.acs.org/doi/pdf/10.1021/jacs.0c03125.
- Huang, X.; Liu, W.; Ren, H.; Neelamegam, R.; Hooker, J. M.; Groves, J. T. Late Stage Benzylic C−H Fluorination with [18F]- Fluoride for PET Imaging. J. Am. Chem. Soc. 2014, 136, 6842−6845. https://pubs.acs.org/doi/10.1021/ja5039819.