Methyl groups are very common in drug molecules. As reported by Heike Schonherr and Tim Cernak1, more than half of the top-selling drugs contains a CH3. A simple substitution of a C-H with a methyl can increase the potency of a compound by more than 100-fold.
The effect of C-H methylation primarily affects the conformation of the original molecule. Based on this reported statistical analysis2 the effect of methyl can be equally positive or negative. However a positive effect could be decisive in a drug discovery program.
Effect of ortho substitution3,4.
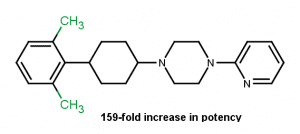
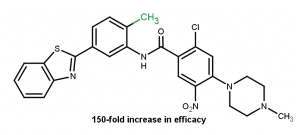
Effect of ring substitution5
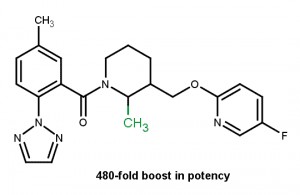
Effect on rotatable bond6
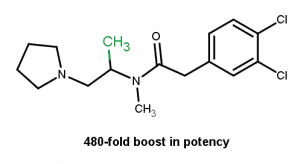
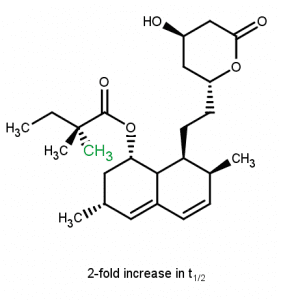
Even if the methyl can be seen as a potential hot spot for metabolism. The addition of the methyl next to a metabolism position can improve metabolic stability.7
Contact us at info@hepatochem.com
- Heike Schonherr and Tim Cernak, Angew. Chem Int. Ed. 2013, 52, 12256-12267
- C. S. Leung, S. S. F. Leung, J. Tirado-Rives, W. L. Jorgensen, J. Med. Chem. 2012, 55, 4489 – 4500.
- F. Berardi, C. Abate, S. Ferorelli, A. F. de Robertis, M. Leopoldo, N.A. Colabufo, M. Niso, R. Perrone, J. Med. Chem. 2008, 51, 7523 – 7531.
- J. Y. Hwang, D. Smithson, F. Zhu, G. Holbrook,M. C. Connelly, M. Kaiser, R. Brun, R. K. Guy, J. Med. Chem. 2013, 56, 2850 – 2860.
- P. J. Coleman and coll. , Chem- MedChem 2012, 7, 415 – 424.
- G. F. Costello, R. James, J. S. Shaw, A.M. Slater, N. C. J. Stutchbury, J. Med. Chem. 1991, 34, 181 – 189.
- R.W. Friesen and coll., Bioorg. Med. Chem. Lett. 1998, 8, 2777 – 2782.

