When screening reactions, matching the optimal wavelength to a photocatalyst requires multiple experiments and setups and a lot of time. Learn about designing Lucent360 customized reaction screenings with multiple wavelengths in one experiment.
With the Lucent360™ you can screen up to 4 four wavelengths independently in one experiment, while also controlling for temperature and light intensity. In this note, we demonstrate how this capability allows you to design a custom chemical screen with the flexibility to match the optimal wavelength for each catalyst and reaction condition.
Customized Reaction Screening
Instrument Configuration
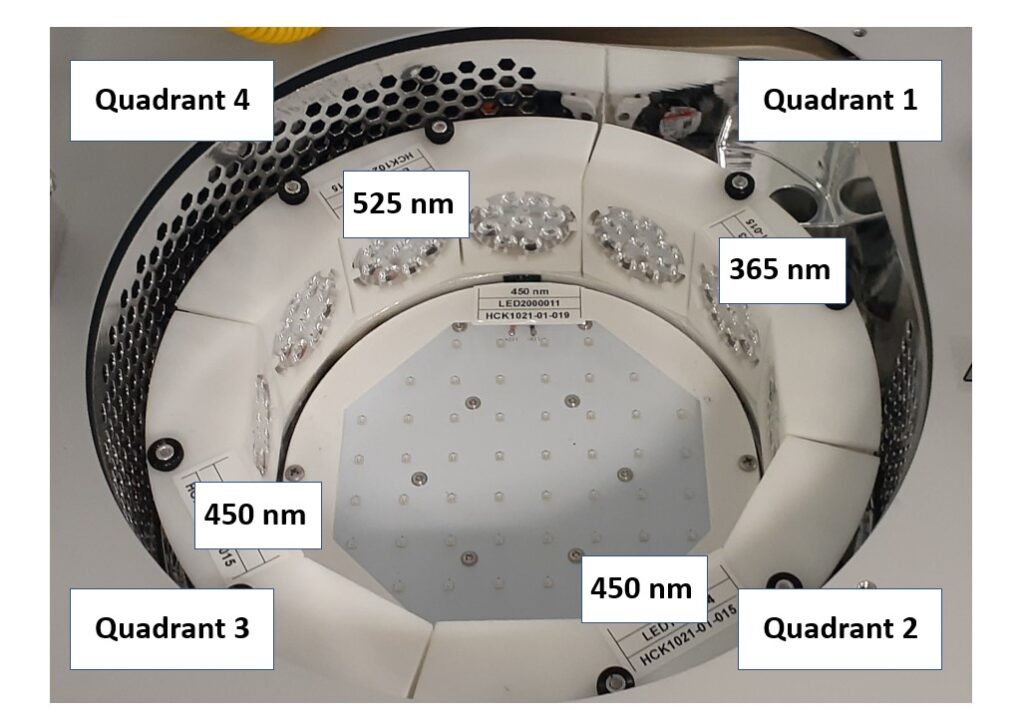
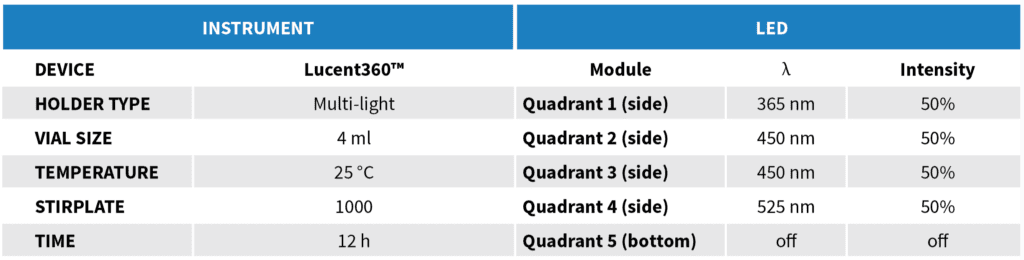
The Lucent360™ includes four side LED modules and one bottom module. Each module is independently controlled and fully interchangeable allowing multiple configurations for wavelength and light intensity.
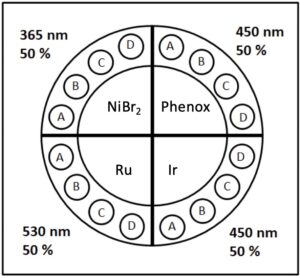
For this example, we will operate the Lucent360™ with four different side modules to screen 365, 450 and 525 nm light. The light intensity for each side module is independently set at 50% and the bottom LED is not in operation for this experiment. Using the multi-light screening holder allows each quadrant to be operated independently without any interaction with the light from the other quadrants. For the 4 ml vial screener, up to 4 reactions can be performed in each quadrant (16 total). This function allows us to design a chemical screen with four unique catalysts each matched to an optimal wavelength for a corresponding C-N bond formation reaction.
The Lucent360™ temperature control unit maintains a constant and controlled temperature at all positions in the array. For this example, we will run all of the reactions at 25 °C. This prevents temperature increases due to irradiation of the LED’s or from internal exothermic processes. The device can be operated from 0-80 °C with the appropriate coolant.
Protocol
Customized Reaction Screening Design
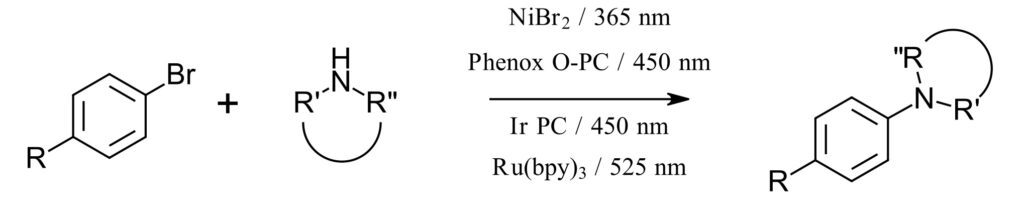
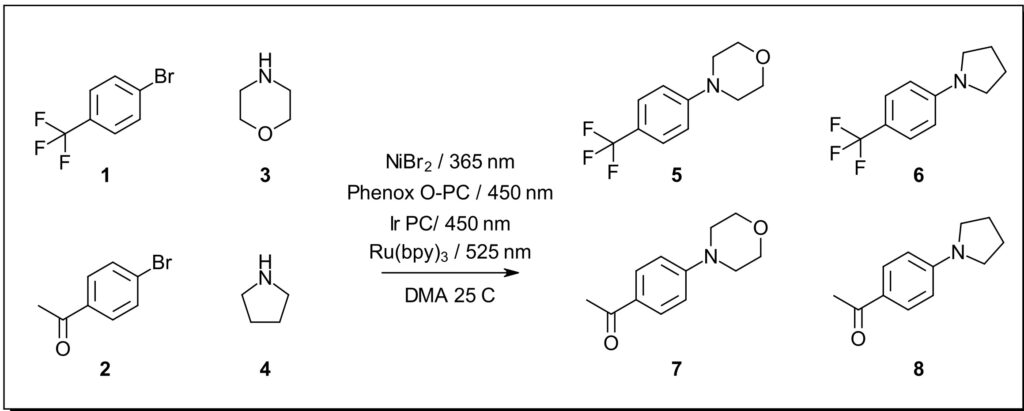
We set out to design a C-N bond formation screen based on known literature examples matched to the ideal LED wavelength excitation for each photocatalyst. The selected transformation occurs between an aryl bromide and secondary amine in the presence of a nickel source, base and a photocatalyst. Each of the four selected conditions uses a nickel catalyst without the need for an additional ligand.
Substrate concentration, scale and catalyst loading were modified from the reported examples to standardize the four selected conditions. Using DMA as the solvent standardizes sample prep for the 4 substrate solutions.
For this screen we selected 2 aryl bromides 4- bromobenzotrifluoride 1 and 4-bromoacetophenone 2 and 2 amines morpholine 3 and pyrrolidine 4 for the potential formation of 4 unique coupling products. The Lucent360™ configuration his affords the opportunity to test each of these potential combinations with each catalyst/wavelength.
Experimental Protocol
In general, each reaction contains 0.8 mmol bromo (1 equiv.), 2.8 mmol amine (3.5 equiv.) , 2 mol % PC, 5 mol % Ni catalyst and if applicable 1.8 equiv. DABCO in 2 ml DMA.
Reaction A: bromo 1 + amine 3 / product 5
Reaction B: bromo 1 + amine 4 / product 6
Reaction C: bromo 2 + amine 3 / product 7
Reaction D: bromo 2 + amine 4 / product 8
The first reaction is a NiBr2-catalyzed reaction using 365 nm LEDs that does not require a photocatalyst as reported by Miyake (Ref 1). The proposed mechanism invokes a Ni-amine-solvent complex that is excited by the 365 nm light. This reaction proceeds without the requirement for any additional base if the amine selected is used with 3.5 equiv. of secondary amine.
The second reaction uses an organic photocatalyst Phenox O-PC™ A0202 (Ref 2) with 450 nm LED and NiBr2. The development of organic photocatalysts as alternatives to traditional Ir and Ru photocatalysts represents an important advance in the long term viability of photocatalysis from both a cost and sustainability perspective.
The third example as reported by Macmillan (Ref 3) utilizes perhaps the most commonly used iridium photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 at 450 nm, NiCl2-glyme and 1.8 equiv. of an additional base DABCO. Use of the additional base allows a lower loading (1.5 equiv. of amine) for the reaction to proceed.
The fourth reaction utilizes Ru(bpy)3, with NiBr2 at 525 nm as reported by Miyake (Ref 2). The authors found that with Ru(bpy)3 as the catalyst, the optimal C-N bond formation reaction occurred at 525 nm.
There are many considerations for why flexibility of catalysts selection and reaction parameters can be important when planning out a reaction screen. Some factors can include the cost of the catalysts, stability in presence of substrates and suitable substrate scope. The flexibility of the Lucent allows for screening optimal conditions in 1 step for whatever type of chemical screen that you can imagine.
Results and Discussion
In a single experiment, the Lucent360™ affords the opportunity to screen 16 unique reaction conditions matching the optimal wavelength and catalyst. In this example, we have four combinations of bromo and amine substrates. Setup of the 16 unique reaction conditions using common stock solutions took approximately 1 hour and the reaction time was set for overnight, although it is possible that many of the conditions reach completion in a shorter time.
The expected products were obtained for each bromo and amine combination. Comparing the results between catalysts showed some interesting results. First, the Ir cat / 450 nm condition gave nearly complete conversion for both reactions using morpholine to give product 5 (99%) and product 7 (94%) which out performed the other catalysts. However, Ir cat/450 nm also gave the lowest conversion that was observed for product 6 (45%) and product 8 (35%) conditions using pyrrolidine.
Phenox-OPC™/450 nm resulted in the best conversion observed for product 6 (86%) and represents a viable metal free alternate for each tested reaction with respectable conversions ranging from 57% to 86%.
NiBr2 / 365 nm gave the highest conversion observed for product 8 (~85%). Due to the lack of need for an additional photocatalyst and the extremely low cost of NiBr2 (less than $1 / g compared to Ir[dF(CF3)ppy]2(dtbbpy)PF6 ($750/g), or Ru(bpy)3 $79/g, or Phenox- PC™ at $1100/g), this reaction is likely the most cost effective condition regardless of slightly higher conversions observed for other catalysts.
Ru(bpy)3 gave respectable conversions for each condition (50-68%) but did not lead for any of the tested reactions. Overall, the screen demonstrates the utility for screening multiple catalysts in a single experiment. It is possible that more variation could be observed between catalysts with a more diverse substrate set or with shorter reaction times.
This custom screens demonstrates just one example of how the flexibility of the Lucent360™ allows for interesting screening possibilities. One could imagine a screen with the Ir catalyst at each different wavelengths. Or multiple amines. Perhaps the light intensity can be optimized for each catalyst. At smaller scale, screen up to 48 reactions with the Lucent360™. The possibilities are endless and the Lucent360™ allows you to design the best screen to fit your needs.
References
(1) Lim, C. H.; Kudisch, M.; Liu, B.; Miyake, G. M. C-N Cross-Coupling via Photoexcitation of Nickel-Amine Complexes. J. Am. Chem. Soc. 2018, 7667-7673. https://doi.org/10.1021/jacs.8b03744.
(2) Kudisch, M.; Lim, C. H.; Thordarson, P.; Miyake, G. M. Energy Transfer to Ni-Amine Complexes in Dual Catalytic, Light-Driven C-N Cross-Coupling Reactions. J. Am. Chem. Soc. 2019, 141 (49), 19479–19486. https:// doi.org/10.1021/jacs.9b11049.
(3) Corcoran, E. B.; Pirnot, M. T.; Lin, S.; Dreher, S. D.; Dirocco, D. A.; Davies, I. W.; Buchwald, S. L.; Macmillan, D. W. C. Aryl Amination Using Ligand-Free Ni(II) Salts and Photoredox Catalysis. Science (80). 2016, 353 (6296), 279–283.
Phenox-O-PC™ A0202 is a registered trademark of New Iridium