When is an apple an apple or when is it an orange?
How should we compare commercial photoreactors? Or better yet, how do we discuss the important details of a photochemical reaction? Answering this question is at the heart of reproducing experimental results. If everyone used the same photoreactor this would all be simple. Uniformity would be good, but progress would likely stall as well.
This is a question that we are reluctant to discuss. If this were a JACS article, we would lead with the disclaimer “The authors declare a financial interest in the selling of photoreactors” but you are reading this on our website, so you already know that. We sell a bunch of photoreactors and commenting on what our users are doing with our reactors or comparing our photoreactors with competitors is complicated. The results are the results when the comparison is fair. But when are we comparing apples? And when do we have an apple and an orange? We’ve alternated being annoyed, indifferent, and inspired to write this piece and have spun back around to inspiration. We went long on this, so skip ahead if you like…
Part 1: What’s important in visible light photocatalysis?
Part 2: Spectrometers, thermometers and actinometers
Part 3: 6 Degrees of (Temperature) Separation
Part 4: “Effects of Light Intensity and Reaction Temperature on Photoreactions in Commercial Photoreactors”
Part 1:
What’s important in visible light photocatalysis?
The number of words reviewing photocatalysis in the last month (although most have been online for a while) is extraordinary . Within this Chemical Reviews issue is a useful “Reference #1” for any flavor of visible light photocatalysis manuscript. And, while we can’t say that you should read every word (if you do, clear a few weeks from your schedule), we recommend this recent perspective in Chem Catalysis by Timothy Noël and Eli Zysman-Colman on “The promise and pitfalls of photocatalysis for organic synthesis.” (Open Access – Ref 1). We echo every word in this piece and appreciate how it is presented. Not to put words in the authors’ mouths but to us, we would summarize this piece as “Let’s all be better!”
Being better means reporting better. With a lamp and a clamp, you can perform a reaction that would have seemed absurd not too long ago. Shoot first, ask questions later. Explaining and describing what you did is another story. Change a few details, some seemingly trivial, like perhaps choosing a larger vial or a new LED and that reaction just might not look the same. We wrote about some of our early experiences developing photoreactors here.
Setting aside the chemicals that are in the vial (a weird place to be as a chemist), what should we know about a photochemical setup? What’s important and what isn’t?
3 Things that are important:
- Light intensity in the specific vial/flask size and shape (well stirred)
- Wavelength
- Temperature (in the vial)
3 Things that aren’t helpful:
- Listing the wattage of your LED
- The color of the LED (Blue LED is insufficient)
- Reporting that the reaction was run with a fan.
Each of these parameters can be better described and need to be accurately reported to understand an experiment. Commercial photoreactors can act as a short cut to a full explanation. Instead of needing to describe a setup with a picture or that the vial was 4 cm from an LED, you can cite a photoreactor. For reproducing experiments, this is successful, as commercial photoreactors are well adopted within industry (Ref 2). And while this standardizes experiments, many will continue with a simpler set up. Just as you can still purify a compound with a glass column and silica gel or use an automated purification system, you would be expected to report the purification conditions equally in depth.
Commercial photoreactors give uniformity to an experiment, but each comes with their own biases, features, and bugs incorporated into important reaction parameters. At its simplest operating principle, a commercial photoreactor (or homemade setup) with its LED, reactor geometry, and vial dimensions is adding a reagent (the photon) to your reaction. While adding light, you are also heating the reaction simultaneously either from the heat emitted by the LED, light absorption of the materials associated with the reactor or via exothermic reactions in your vial. This can be a feature (the reaction needed some heat anyway) or a bug (I really wish my reaction was just a little bit cooler or more stable). Understanding these details is the first step towards comparing reactors.
Part 2:
Actinometers, Spectrometers, and Thermometers
As part of an internal project (expect more on this soon), we dove into the literature citations using our EvoluChem™ PhotoRedox Boxes. Based on how our reactors are cited, in both the text and figures, we’ve surely missed a few (send us your papers!). We found nearly 100 examples that we were able to access so far using our PhotoRedox Boxes. From this review, there are a few general themes for how our reactors are used:
- A PhotoRedox Box with an EvoluChem 18 W LED (wavelengths varying from 365 nm to 740 nm), or EvoluChem 30 W LED (365 nm or 450 nm)
- Our box with a variety of Kessil™ lamps
- Our box with the fan turned on or off to maintain or increase temperature
- Modifications to fit different things inside (flow tubing, NMR tubes, specialize gas-tight cylinders
The flexibility of the PhotoRedox Box means that you can put our LEDs or other companies LEDs in the reactor, use any vial size from 200 μl to 20 ml and then cite vaguely the “EvoluChem PhotoRedox Box”. We are grateful that users find a way to use our box to fit their needs (Innovation!) but it does mean that the light intensity will vary from experiment to experimental setup.
Actinometry:
Photons are a reagent, but you only get to use the photons that get from your LED into your flask. The wattage of your LED doesn’t matter. However, light is difficult to accurately measure, especially within a 3-D object like a reaction vial. A light probe placed in the position of the reaction reporting watt, lumen, or lux (as a proxy for light intensity) will give you the same reading whether the vial you are using 2 ml or 20 ml or a 200 ml flask. We can and have stuck a light probe into a PhotoRedox Box but that value is close to meaningless, measuring one direction of light instead of a 360 degree view. To generate a light intensity value of any use, we need to use actinometry. A detailed discussion of the perils and advantages of different actinometric methods is due a full post on its own but two things are certain. Most actinometry methods are time consuming and complex and new methods are needed for high energy light sources. (Ref 3) For our work, we use the ferrioxalate method that we discuss here but are looking to improve our methods.
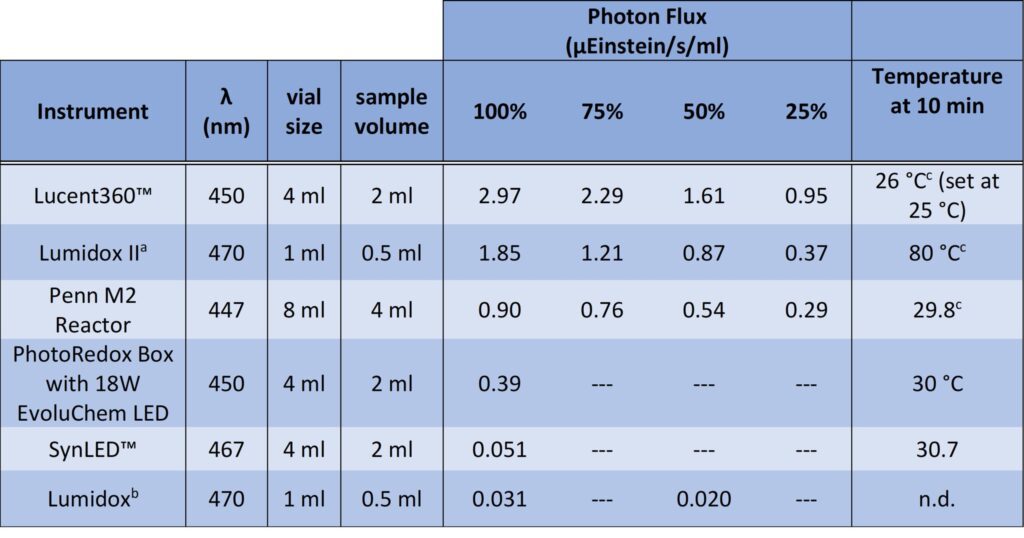
We have investigated several commercial photoreactors including our own, to better understand the light intensity present in each. Each instrument was used with a sample volume fitting of its general use. And we’ve reached our first issue with comparing commercial reactors. The actinometer values present hold true only for this specific volume and vial. Change the vial size, volume or light source and you need to repeat the actinometry. We report photon flux as μEinstein/s/ml to best align with what is happening at the level of a chemical reaction. To put this another way, this is μmol photons/s/ml.
Table 1: Actinometry results for select commercial photoreactors
a. Settings 650 mW, 460 mW, 350 mW, 120 mW. Temperature reaches 80°C in 10 min
b. Settings at 30 mA and 20 mA
c. Temperature recorded at 100 % light intensity setting for each instrument
Wavelength:
Not all LEDs are created equal. The emission spectra can vary greatly between type of LED. For each of our LEDs, we report the specific spectrum our LEDs (Figure 1). Running a reaction with a narrow band LED is best. Try a few of them, you might find some interesting wavelength effects on your chemistry. Running a reaction with a white LED as anything other than an initial screen seems weird to us, but to each their own.
Figure 1: EvoluChem Light Spectrum 365 to 740 nm LEDS including White 6200K

Temperature:
For the 18 W EvoluChem LEDs with the PhotoRedox Box, the temperature measured in a vial position will give a stable temperature around ~29-30 °C during an extended run time. With a Kessil light in the PhotoRedox Box, the temperature is ~1-2 °C higher. Stick something else on the box, and you’ll need to measure the temperature for yourself for comparison. In a reaction with a highly absorbing material, then you are likely heating up your vial internally as well and measuring the temperature in the vial will be important to understanding your reaction. Both the PhotoRedox TC (temperature controlled) and Lucent 360 allow active cooling with circulating coolant and the temperature can be maintained at any temperature between 0°C to 80 °C. For a brief survey of the operating temperatures that we have observed with other photoreactors, see Table 1 above.
Part 3:
6 Degrees of (Temperature) Separation
Temperature, wavelength, and light intensity. Why do all these details matter? We have been fascinated by this work by Dixon, et. al. “Switchable, Reagent-Controlled Diastereodivergent Photocatalytic Carbocyclisation of Imine-Derived α-Amino Radicals” (Ref 4) since we read it last year. We included it in our year end piece on 21 papers from 2021. The title reaction (Figure 2) demonstrates a redox controlled α-amino radical intermediate with adjacent alkenes that can either undergo: (A) a net reductive single stereoselective cyclization to give trans amino-indane structures, or (B): undergo two consecutive radical cyclizations to give tetracyclic tetrahydroquinoline structures (B).
Figure 2: “Switchable, Reagent-Controlled Diastereodivergent Photocatalytic Carbocyclisation of Imine-Derived α-Amino Radicals” (Ref 4) (For the purposes of our discussion we’ve modified Scheme 2 and data from the Supporting Info).

The authors describe an extensive optimization process to arrive at these conditions, proposing the choice of Hantzsch ester to play a key role in conjunction with solvent and concentration effects. With HE1, the stronger reductant, favoring the net reductive trans single cyclization while milder reductant HE6 results in consecutive cyclizations. Each of these 2 conditions is highly selective towards 1 of the pathways, with an extensive array of conditions falling somewhere in between. The full scope of the paper is impossible to summarize in one short blurb, so if you are interested in these type of things then please read the full paper.
Table 2: Selected results for conditions using in situ prepared imine including 20 mol % AcOH, R=OMe

What piqued our interest (in addition to the chemistry) is that this work is described in the Supporting Info as taking place at two sites, with two different reactors, our EvoluChem Photoredox Box and the SynLED (Table 2). A situation, which if handled poorly, could result in some inconsistent and misunderstood results. And yet, the authors took the time to do a thorough detailed look at the differences in product formation over many parameters including factors associated with each reactor.
Now, this may be a detail that only someone with a personal interest would notice or even make it to page 53 of the supporting information, but we were intrigued by this reaction. For the reaction as described in Figure 2 (R=H), the authors looked at temperature effects using the PhotoRedox Box with a flow gas to lower the temperature to 23 °C and with the fan off to reach 40 °C (Marketing note: this could also be accomplished the PhotoRedox Box TC over a larger temperature range in an easier manner – back to the science)
Table 4: Adapted from (Ref 4) page 53 Supporting Info and modified for clarity, Reaction Figure 2, preformed imine, R=H.

Our takeaways:
- At 23 °C, with careful optimization of reagents, selectivity can swap between 10:1 and 1:10
- At 29 °C (only 6 degrees higher) and the result is a lot less exciting
- At 40 °C, all the careful optimization of reagents and conditions is lost and conversion is significantly lower
- Did you notice what happens if you don’t stir the reaction?
From this the authors support the main table result that small temperature differences play a role in the selectivity difference observed between the SynLED and the Photoredox Box, while also noting the lower light intensity of the SynLED. A direct comparison of the SynLED and Evoluchem at 23 °C with identical reactions conditions wasn’t found in the SI. Are we intrigued by what the difference in light intensity might show if temperature is also controlled based on what we know about the difference in light intensity. Yes, we are. Did we leave out the discussion of the many other factors at play in the differences between photoreactors, including vial size, wavelength and time course that may add another layer of complication? Yes. Do we think the authors did the best they could at describing, right in the main table example important details of the work. We do.
Part 4:
Comparing Commercial Photoreactors
So, burying the lede. The article that got us into our cycle of annoyance, indifference, and inspiration was this article “Effects of Light Intensity and Reaction Temperature on Photoreactions in Commercial Photoreactors” (Open Access – Ref 5). The authors chose six photochemical reactions relevant to medicinal chemistry from literature (more on these in a bit) to run in four commercial photoreactors. Some of the results involving our PhotoRedox box surprised us, and in our view often weren’t presented in the best light (pun intended). We would let this all go without a response; except we’ve been asked several times if we’ve seen this article. Yes, we have. And since we have a lot of thoughts on this article and some additional data to add, we’d like to share it. For the purposes of full disclosure, we have shared with the authors the data, and comments that we add here.
When we first saw this title, we were intrigued by the idea because you may have heard, we sell photoreactors. And we agree with the main aim of the paper:
“The aim of the comparison is to illustrate that different photoreactors will lead to different reaction performance dependent on the reaction performed and to highlight that light intensity should not be the only factor considered when optimising the yield and reaction times of photoredox catalysed transformations.”
Do we expect to see differences between photoreactors? Yes, we do.
When we see differences, what does it mean? This is much more difficult to answer.
Introducing our participants:
The four photoreactors examined were the PennOC Photoreactor M2, EvoluChem PhotoRedox Box, TAK120 AC (air-cooled) and TAK120 LC (liquid-cooled). For each reaction, the TAK120 LC was tested at two temperature settings. In Table 5, we have attempted to summarize as best as we can the key operating parameters in the setup of each reactor for this study. The Penn OC Photoreactor M2 was run at 100% light intensity with the fan at full power. For the PhotoRedox Box, while the picture with the text shows a PhotoRedox box with an EvoluChem LED, the experimental data shows that the reactions were performed with a Kessil 456 nm LED. Selfishly, we would have liked to see the reactor comparison performed with our EvoluChem 18 W LED, as the reactor design was optimized with this LED. We have demonstrated higher light intensity for the EvoluChem LED in the PhotoRedox box than the Kessil due to beam angle and mirror geometries but this is acceptable. The TAK120 are listed as running at 50% intensity to better control the temperature. We present these subtle choices to show that at each stage when comparing commercial photoreactors, decisions are made that will influence the results of the experiment.
Table 5: Key parameters of the instruments (summarized for clarity from Ref 5 with information from main text and Supporting Info.)

*configuration used for this study with a Kessil lamp and 6 vial holder.
The wavelength of each reactor is close enough between 450-456 nm that any wavelength effects should be minimal, although full spectra of the LED emissions are not presented.
As a proxy for light intensity, the authors determined optical power using a thermopile sensor placed in the position of the vial for each reactor. This is not ideal. If the reactor designs were comparable, then this could be a (although not great) proxy for light intensity. However, the TAK120 style is illuminated from below while the reaction sample in both the PennOC and PhotoRedox box are illuminated from all directions. From our previous measurements with actinometry, we expect the PennOC to be about 2x higher light intensity than the Photoredox Box setup, a value not demonstrated by the optical reading. We don’t have access to either of the TAK reactors to do actinometry for comparison and can offer no comment. This is all before taking into account the solvent/surface area differences that might be present from the different geometries and choice of scale for these experiments.
The authors do recognize this issue here: “We are aware that a holistic description and control of a photocatalytic reaction set-up would require considering more parameters than we can address here, such as temperature dependent light emission spectra, the photon flux depending on the intensity setting determined via actinometry, and the light uniformity in the reaction vessel derived e. g. via simulations.”
Of that list, in our opinion only an actinometry analysis of each reaction would have greatly improved the understanding of the difference in light intensity for these specific experiments. For this reason, from our point of view for interpreting the results the best we can do is state in terms of light intensity TAK120 > Penn OC M2 > Photoredox Box. How much different is difficult to say.
A few examples:
So, let’s get to the 6 reactions that were studied. The authors look at a variety of reactions with different radicals and coupling partners to give a diverse set of conditions. Product yields and reaction temperature were determined for a relevant time course for each of the 6 reactions and 4 reactors. An impressive of amount data to assess for each reaction, showing the difficulty and extensive work needed to compare multiple photoreactors. We won’t go into the data for all 6 reactions in each for the five reactor conditions. If you are interested in this sort of thing, we suggest you dig into this paper (and if you made it this far, you might be). We’ll do our best to present our interpretations of the data and how we sometimes view things from a different lens than how the results are presented. For a few of the reactions where we were able to both access the reagents and do the work inexpensively, we can add additional results.
6 Reaction Types studied:
- Brønsted Acid Photocatalytic Radical Addition of α-Amino C-H Bonds Across Michael Acceptors
- Trifluoromethylation of Arenes and Heteroarenes by Photoredox Catalysis
- Regioselective Amination of Arenes Using Alkyl Amines
- Organo-Photoredox Minisci Reaction Using N-(Acyloxy) phthalimides
- Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis
- Selective sp3 C-H Alkylation Via Polarity-Match Cross-Coupling
Overall, for this set of experiments a few general trends emerge. For each photoreactor, the experiments done in triplicate are highly reproducible demonstrating a key feature of using a commercial photoreactor. However, when run in this manner, each photoreactor is an intractable sum of its parameters. When thinking about the results, we can group our understanding of the data points in the following manner:
Photoredox Box: lowest light intensity / lowest temperature (consistently 30 °C) / stable temperature throughout the run
Penn OC M2: Medium (to highest) light intensity and higher temperature (consistently 44 °C), stable temperature through the run
TAK120 AC: Highest light intensity / highest temperature / uncontrolled temperature
TAK120 LC (25 °C): Highest light intensity / observed temperature ~10 °C higher than setting (~34 °C)
TAK120 LC (35 C): Highest light intensity / observed temperature ~ 10 °C higher than setting (~44 °C)
With this in mind, let’s look to see what we conclude about this data set in regards to temperature and light intensity.
EXAMPLE 1: Brønsted Acid Photocatalytic Radical Addition of α-Amino C-H Bonds Across Michael Acceptors
The first comparison from the paper that we want to discuss is the Bronsted acid photocatalytic radical addition ofα-amino C-H bonds across Michael acceptors as shown in Figure 3. To best summarize each set, we have modified the data tables to include both temperature and yield.
Figure 3: Bronsted acid photocatalytic radical addition of α-amino C-H bonds across Michael acceptors

Table 6: Comparing 5 photoreactor setups for the Bronsted acid photocatalytic radical addition of α-amino C-H bonds across Michael acceptors

- The first point that the authors present is that each reactor showed an improvement in yield at 90 minutes compared to the literature example (<50%)
- The second point that we will highlight is that the temperature varies greatly in the experiments (a nearly 48 °C range in temperature between the PhotoRedox Box and the TAK120 AC)
- For the three TAK120 -highest light intensity reactions (blue square) the initial rate is the highest, although all three flatten quickly
- The TAK120 AC reaches a nearly unsafe uncontrolled temperature rather quickly, while showing similar results to the TAK120 operating 40 °C cooler
- Ultimately the highest conversion was observed for the Penn OC reactor, stated as the middle point in terms of light intensity and temperature (green box)
- Combining the data and plots from the paper show something interesting to us (Figure 4). The yield for both the Penn OC M2 (orange dots) and the PhotoRedox Box (dark blue dot) appear to still be going up while the higher intensity examples have stalled? Where will the slow/steady and stable reactions ultimately end up? Without following the reaction to completion it is difficult to know. From the data you could make the argument that the majority of the yield for the TAK120 reactors occurs before the reaction reaches what ultimately is the highest temperature before stalling
Here our conclusion can best be described as temperature and light both play an effect on the results.
Figure 4: Plotting time course for Bronsted acid photocatalytic radical addition of α-amino C-H bonds across Michael acceptors.

EXAMPLE 2: Trifluoromethylation of Arenes and Heteroarenes by Photoredox Catalysis
The second example to highlight is the trifluoromethylation of lidocaine (Figure 5). To best summarize each set, we have modified the data tables to include both temperature and yield (Table 7).
Figure 5: Photocatalytic trifluoromethylation of lidocaine

Table 7: Results from Photocatalytic trifluoromethylation of lidocaine

- Here the 3 TAK120 reactors show significantly higher initial yields
- Ultimately the reaction for theTAK120 at 44 °C goes nearly complete to conversion while the other two TAK120 examples do not. This suggests the possibility that while light intensity is important, there also might be a sweet spot in temperature ~40 °C with too hot and too cold detrimental to the yield
- The low conversions observed in the PhotoRedox Box confused us here, as we have run similar experiments
We used the tools we have at hand to probe this reaction a little further. With, the (a) PhotoRedox Box/EvoluChem 18 W 450 nm LED, (b) a PhotoRedox Box/Kessil 150 and a PhotoRedox Box TC/ EvoluChem 18 W 450 nm LED we can look at light intensity and temperature (Table 8).
- In our hands, this reaction shows significantly higher conversion than observed in the publication
- With identical light sources, we observe identical initial yields at 30 °C and 50 °C. However, at higher temperature, the reaction stalls while continuing at lower temp
- The lower intensity Kessil light give a lower yield than for the EvoluChem LED
- We have no explanation for the low conversion observed in this paper for the Photoredox Box as the results we observe are very different, even with a Kessil LED. Perhaps a subtle experimental difference in initiation of the radical reaction at lower temperatures is to blame? We have many thoughts and theories on this but won’t bore you any further
Table 8: Unpublished HepatoChem results from Photocatalytic trifluoromethylation of lidocaine

EXAMPLE 3: Regioselective Amination of Arenes Using Alkyl Amines
The third example to describe is the Regioselective Amination of Arenes Using Alkyl Amines (Figure 6). The literature example of this reaction is run at 0 °C and likely represents a challenge for commercial reactors that cannot achieve below ambient temperatures. The original paper gave 67% conversion for the reaction in question. Presumably, this reaction would demonstrate a temperature effect with the TAK120, able to operate at 0 °C. As expected, the only reactor to result in significant conversion was the TAK120 LC., although only reaching 31%. (Table 9) This result would suggest that low temperatures are indeed important but that increased light intensity was not productive.
Figure 6: Regioselective amination of arenes using alkyl amines

Table 9: Regioselective amination of arenes using alkyl amines

While we cannot comment on why the TAK120 did not reproduce the high yields from the paper, the authors suggest that rising 5 degrees higher than the desired reaction temperature may play a factor. As a comparison, we ran this reaction in the Photoredox Box TC which has an identical layout to the PhotoRedox box but can be operated at 0 °C with an external chiller. In addition, we ran this reaction with our Lucent360 which can control both temperature and light intensity. We ran this example reaction in the Lucent 360 at 4 light settings as well as in the PhotoRedox Box TC with EvoluChem 18W LED (Table 10). Both experiments were able to maintain 0 °C through the course of the reaction. With high powered Lucent 360, the reaction was complete in 15 minutes and we started to observed over-addition of the amine.
Table 10: Unpublished results using the Lucent 360 and Photoredox Box TC at 0 °C.

REMAINING EXAMPLES:
For the Organo-Photoredox Minisci Reaction Using N-(Acyloxy) phthalimides, ultimately all 5 reactors gave similar yields between 70-90% with slightly reaction profiles. Each exceeded both in conversion and reaction time the literature examples. Similarly, for the 5th example Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis all 5 reactors yields were between 71-80%. The 6th example, Selective sp3 C-H Alkylation Via Polarity-Match Cross-Coupling ultimately the Penn OC M2 and TAK120 AC gave the highest yields 54-55%, followed by the PhotoRedox Box (43%) then the TAK120 LC at 29-34% suggesting something about light intensity and temperature.
Summary:
Overall, light intensity and temperature have an effect on photochemical reactions, but we struggle to feel too strongly about any broad conclusions when comparing reactors. Do temperature and light intensity matter in Photocatalysis? Unequivocally Yes. Do we expect that any reactor type will have a consistent trend for yield based on light intensity and temperature for mechanistically diverse reactions that exist in visible light photocatalysis?
- Photoredox
- Hydrogen atom transfer
- Proton coupled electron transfer
- Metallophotoredox
Probably not. We also expect a photoreactor comparison might show entirely different results for the identical reactions with subtle stoichiometric differences like higher or lower catalyst loading, stirring or solubility of reagents, as those factors interact with more or less light, higher or lower temperature and reactor geometries. Will a reactor that can control both temperature and light intensity be more useful for investigating these mechanisms, yes of course. And really, our problem with this entire article can be boiled down to this simple idea. Is comparing reactors a thing we need to do, or should we compare reactions with a better understanding of the parameters in the reaction? When the temperature and the light intensity cannot be independently controlled for, or understood in a manner that can be controlled, then we have apples and oranges. For the choice of any commercial photoreactor, really should come down to several key points.
- Are reactions reproducible?
- Is the reactor flexible to run multiple wavelengths?
- Can I run the number of reactions that I need in an acceptable amount of time?
- Do I understand the reaction parameters in manner that I can scale up the reaction?
If so, then the reaction setup is a success.
References:
- Noël and Zysman-Colman, The promise and pitfalls of photocatalysis for organic synthesis, Chem Catalysis (2021), https://doi.org/10.1016/j.checat.2021.12.015
- Candish, L.; Collins, K. D.; Cook, G. C.; Douglas, J. J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122 (2), 2907–2980. https://doi.org/10.1021/acs.chemrev.1c00416.
- Kuhn, H. J.; Braslavsky, S. E.; Schmidt, R. Chemical Actinometry (IUPAC Technical Report). Pure Appl. Chem. 2004, 76 (12), 2105–2146. https://doi.org/10.1351/pac200476122105.
- John, A.; Paul, A.; Leitch, J. A.; Yamazaki, K.; Christensen, K. E.; Cassar, D. J.; Hamlin, T. A.; Dixon, D. J. Switchable, Reagent-Controlled Diastereodivergent Photocatalytic Carbocyclisation of Imine-Derived α-Amino Radicals. Angew. Chem. Int. Ed. 2021, 60 (45), 24116–24123. https://doi.org/10.1002/anie.202107253.
- Svejstrup, T. D.; Chatterjee, A.; Schekin, D.; Wagner, T.; Zach, J.; Johansson, M. J.; Bergonzini, G.; König, B. Effects of Light Intensity and Reaction Temperature on Photoreactions in Commercial Photoreactors. ChemPhotoChem 2021, 5 (9), 808–814. https://doi.org/10.1002/cptc.202100059.