In recent years, amide coupling has become the most frequently used reaction in medicinal chemistry¹. Found as the backbone of proteins, the amide bond is nominal formed by the condensation of a carboxylic acid and an amine. Due to a nearly unlimited set of readily available carboxylic acid and amine derivatives, amide coupling strategies can be an efficient approach for medicinal chemists to generate novel compounds. As a result of this utility, a nearly unlimited set of reagents and protocols have been development to afford this simple transformation of amide bond formation².
Amide Bond Formation

Amide Coupling Kits
- Carbodiimide Reagent Kit
(HCK1005-01-002) - Aminium Coupling Reagent Kit
(HCK1005-01-004) - Phosphonium Coupling Reagent Kit
(HCK1005-01-006) - First Choice Kit
(HCK1005-01-007)
The most common method for formation of an amide bond is the condensation of a carboxylic acid and an amine. Generally, the carboxylic acid needs to be activated in order to react with the amine while remaining reactive functional groups need to be protected. This process occurs in two steps in either one pot with direct reaction of the activated carboxylic acid or in two steps with isolation of an activated “trapped” carboxylic acid with reaction with an amine.
Two step peptide bond formation
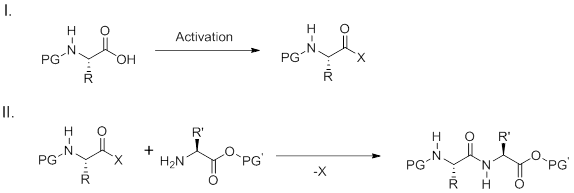
The carboxylate reacts with the coupling reagent yielding a reactive intermediate which can often be isolated or used immediately with an amine to form an amide bond. A wide variety of reagents have commonly been used to generate the activated carboxylic acid such as an acid halide (chloride, fluoride), azides, anhydrides, or carbodiimides. Additionally, active esters such as pentafluorophenyl or hydroxysuccinimido esters can be prepared as reactive intermediates. Reactive intermediates derived from generation of acyl chlorides or azides are highly efficient for amide coupling; however, their harsh formation and high reactivity often limits use with complex substrates or amino acids. A broadly applicable method for the formation of amide bonds use carbodiimides such as DCC (dicyclohexylcarbodiimide) or DIC (diisopropylcarbodiimide) for activation. Additives are often required to improve the efficiency of the reactions especially for solid-phase synthesis.
To avoid side reactions involving the substituents on the two coupling components, it is often necessary to carefully select the appropriate peptide-coupling reaction condition. One common problem with the use of carbodiimides is the racemization of amino acids. To remedy this, two classes of coupling reagents: Phosphonium and aminium reagents represent significant improvements over carbodiimide methods.
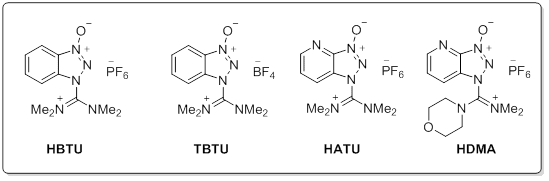
Common Aminium reagents
Common Phosphonium reagents
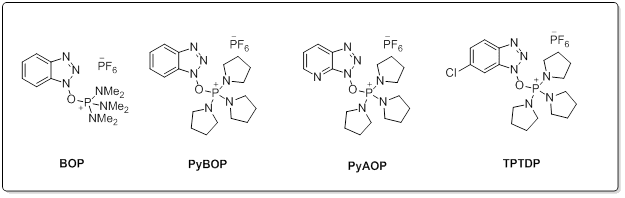
Aminium salts are very efficient peptide coupling reagents with quick reaction times and minimal racemization. With the addition of an additive such as HOBt, racemization can be completely eliminated. Aminium reagents are used in equal molarity to the carboxylic acid to prevent excess reagent reacting with the free amine of the peptide preventing coupling. Phosphonium salts react with carboxylate requiring usually 2 equivalent of base. (such as DIEA). One key advantage for the use of phosphonium salts over iminium reagents is that phosphonium does not reactive with the free amino group of the amine component. This allows couplings to occur in equimolar relation between the acid and amine, highly advantageous in situations such as the intramolecular cyclization of linear peptides or examples where excess of valuable amine component is discouraged. Choosing the correct reaction for your needs: While amide bond formation is a straightforward reaction, the choice of suitable reagents for an individual coupling of a complex carboxylic acid and amine may be a difficult decision. A variety of factors can be involved in finding the optimal peptide coupling reaction. Unanticipated side reactions or rearrangements, low conversions, solubility issues, or incompatibility with additional synthetic steps can all hinder what would appear to be a straightforward amide coupling reaction. As such, it is often necessary to draw from the large number of available reagents and protocols to find the optimal reaction condition. Screening a selection of reagents can often find a suitable reaction for any amide coupling.
References:
1. Brown, Dean G., Bostrom, Jonas, “Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?” J. Med. Chem., 2015, ASAP.
2. El-Faham, Ayman; Albericio, Fernando, “Peptide Coupling Reagents, More than a Letter Soup” Chem. Rev., 2011, 111, 6557. 2. Pattabiraman, Vijaya R., Bode, Jeffrey W. “Rethinking Amide Bond Synthesis” Nature, 2011, 480, 471.

