For this month, bring on the red light! Here at Hepatochem, we’re intrigued by examples that we’ve seen recently using red LEDs for synthesis and wanted to highlight an interesting paper in this area. (We’ve also noticed an uptick in the sale of LEDs in this region as well, so there is clearly an interest out there in red light photochemistry.) We first noticed red light photochemistry a few years ago and wrote about osmium photocatalysis in one of our earliest blog posts. Since then, a few red-light initiated transformations that have caught our eye include applications for C-N cross coupling, olefin metathesis and protein labeling. So why red light? Let’s discuss.
The benefit of switching to red light can be substantial, if you can still do your transformation with lower energy than you might be accustomed to in the near UV and blue region. And while we’re not a fan of the consequences when things shift from Blue to Red, in photochemistry the results can be advantageous. Red light can offer deeper penetration in certain media (such as skin or some polymers) has a higher percentage in sunlight (if you’re into the potential of that sort of thing), is safer and lower in energy. In several instances a switch to lower energy red light helped to eliminate unwanted photodegradation side products.
In their present work “Synthesis, Characterization, and Catalytic Activity of Ni(0) (DQ)dtbbpy, an Air-Stable, Bifunctional Red-Light-Sensitive Precatalyst” published recently in JACS, Dong Xue and coworkers at Shaanxi Normal University bring nickel photocatalysis into the red, specifically nickel(0) catalysis. Nickel catalysts are quite familiar in photochemistry as numerous examples exist combining nickel and iridium catalysts with blue LEDs (see in any chemistry journal from 2016 to 2025) or on their own like one of our favorite papers by Miyake and coworkers for cross coupling chemistry, usually with something purple (365 nm). But to find a nickel catalyst suitable for work in the red region, first the authors needed to make a new catalyst.
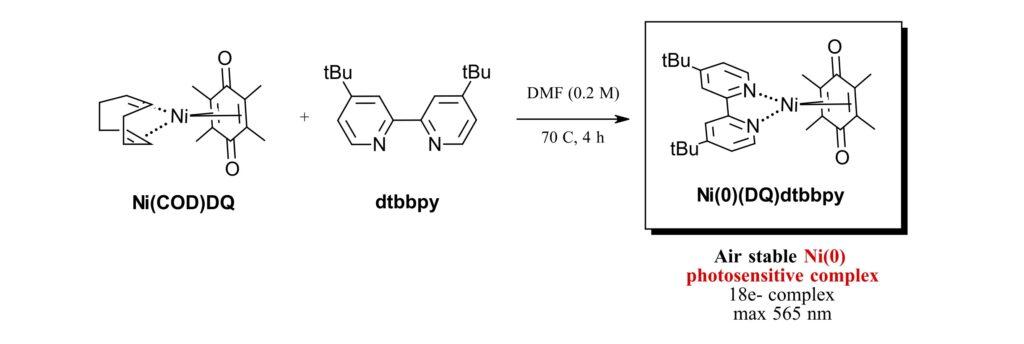
Figure 1: Synthesis of Ni(0)(DQ)dtbbpy
Nickel(0) catalysts are well known in thermal catalysis; however, to date a nickel(0) precatalyst has not been known for photocatalysis. And so, the authors first looked to make their new catalyst. Starting from Ni(COD)DQ with dtbbpy, the authors prepared Ni(0)(DQ)dtbbpy in one step in 84% yield (Figure 1). They fully characterized their new catalyst including UV spectroscopy, CV and X-Ray crystallization. The new catalyst is air stable for several days, demonstrates an absorption band at 565 nm and can undergo 1 or 2 electron processes.
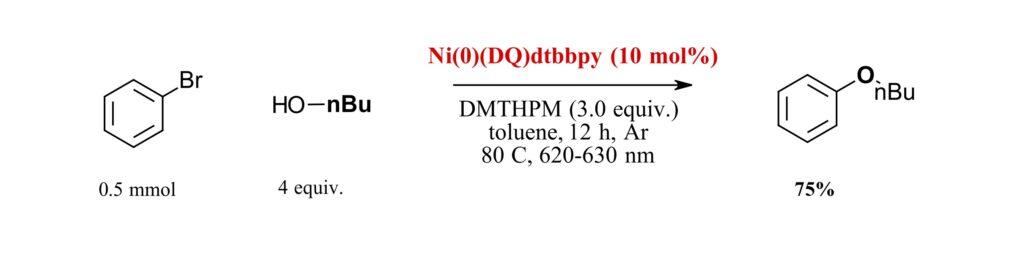
This new Ni(0) complex catalyzes reactions that could not be performed with the original Ni(COD)DQ or other Ni(0) catalysts. The initial test reaction for Ni(0)(DQ)dtbbpy involved the C-O coupling of bromobenzene and n-butanol. Utilizing toluene, red light and a base (DMTHPY) with some heating to 80 °C gave conversion at 75% (Figure 2). Switching out the nickel catalyst gave no reaction as did removing the base. Heating the catalyst to 85 °C in an oven for 4 hours prior to use gave no difference in reaction showing the stability of the catalyst, while storing the catalyst for 5 days in air showed minimal detrimental effects. However, lowering the temperature to 60 °C gave no reaction while the reaction without light but heating at 80 °C gave 10% conversion. The presence of air lowered the conversion to 58%.
Figure 2: Optimizing Reaction condition
The optimized condition was applied to various meta and para substituted aryl bromides including acetyl, cyano, halogen, tert-butyl and trifluoromethyl groups and was extended to heterocyclic systems as well. The reaction was successful for a variety of alcohols and extended to C-N coupling with a variety of amines. Switching to red light afforded a few key advantages, mainly low dehalogenation of substrates, low absorption of light by other reagents and limited nickel black formation. Overall, this system afforded gram scale synthesis. Further mechanistic studies and DFT analysis investigated factors associated with the photochemical and thermal aspects of the catalytic cycle showing the role both play in this catalytic cycle.
This work further demonstrates that there is more to photochemical life than blue and purple. Finding the right combination of catalyst, wavelength and reaction media can find the ideal use for an application. At scale, consider the cost difference between a reaction with an expensive iridium catalyst and blue LEDs verses cheap nickel and potentially sunlight. Overall, a nice move in the direction of increasing the flexibility of Ni photochemistry. Now, lets work on that operating temperature for this system….