Part II: Understanding and Measuring Light Sources
This is the second part of a three part series designed to help you get started by understanding light sources in photochemistry. Missed the first part of the series where we cover the basics and core principles? No worries, you can read it here…
The ease in setting up photochemical reactions led to a rapid adoption of photoredox chemistry. Much of the early photoredox research discussed in Part 1 was performed using readily available, commercial CFLs, flood lights, household light bulbs or low energy LED strips. Often reactions were cooled (or not) with an external fan in an attempt to keep the temperature low from the heat of the light source or generated from the reaction. Unfortunately, little was reported or understood at the time for the wavelength and intensity of light in the reaction flask. Eventually, higher energy, single wavelength LEDs became the light source of choice for most chemists but details on the light used for reactions remained sparse. Often, the prevailing criticism of photochemistry is that a small-scale reaction works but scaling up is impossible. This can be directly attributed to two factors, not knowing how much light is available from your light source and the light being absorbed by your catalyst (more on this at a later date) (Ref 12).
Recently, there has been a concerted effort to treat the light used in the reaction with the same care and focus as you would any stochiometric reagent in a reaction (Ref 15) Not reporting the details of the light being used in your reaction is the equivalent of saying you “heated the reaction” without reporting a temperature. However, determining the intensity and type of light that makes its way into a reaction vial is more complicated than you might think. Part of this is due to the difficulty in how we historically discuss the brightness and intensity of light for commercially available light sources. The second problem derives from making 2-dimensional measurements of light to mimic a 3-dimensional reaction. (fine for the light on a solar cell, not as great for a reaction flask) (Ref 16).
Light is generally divided into three classifications, ultraviolet wavelength (100 to 380 nm), visible light (380-700 nm) and infrared (greater than 700 nm). Sunlight itself is a combination of all of these. The radiation that reaches earth from the sun is a wide collection of wavelengths, ranging from 100 nm to 1 mm. Almost everything below 280 nm is blocked by the earth’s atmosphere (for now), while the collection of wavelengths in the visible region we perceive as white light. The longer infrared wavelengths (heat radiation) make up about 50% of the radiation that we receive from the sun. Similarly, commercial household light bulbs have sought to mimic the white light that we perceive in nature.
The units that we commonly use to describe the brightness (or intensity of light) – whether sunlight or household light sources – find their origin in sunlight and how we perceive it with the human eye. When looking to determine “brightness” of sunlight, we want to know the sum of the all the wavelengths and total energy that is dispersed over a large area. Luminous flux is the measure of the total quantity of visible light emitted by a source weighted according to human eyes sensitivity to various wavelengths (measured in lumens). The unit lumen is the amount of light emitted by a source per unit time. In other words, lumens represent the amount of visible light generated by the bulb or the sun or whatever you are measuring. A lux meter is then used to measure the amount of light in a specific position over a certain area (lux = lumen/m2).
Most light sources report color (wavelength) and electrical power (wattage). The electrical power rating is the indication of that light’s power. Nearly all commercial bulbs are rated in lumens, a unit that averages the full spectrum of light. Monochromatic LED’s make these measurements irrelevant. A CFL and LED with the same power rating will not have the same luminous efficacy and will not deliver the same amount of energy to a reaction. Additionally, household light bulbs diffuse light in all directions while focused light sources such as LEDs focus light in one direction. Variance in beam angle between different types of LEDs further complicate the amount light that ends up in your reaction vial (see Figure 7 below).
For LEDs, radiant flux (measured in watts which are J/s) and light intensity (irradiance, mW/cm2) give us a more accurate measurement. We can use a radiospectrometer to measure an LED’s power (in watt) and light intensity (irradiance in watt/cm2) at a specified distance from the light source, as well as determine the wavelength. Irradiance is measured at a single point in one direction so it can be used to directly compare different light sources. Irradiance and lux are not equivalent as irradiance is not based on the human eye sensitivity.
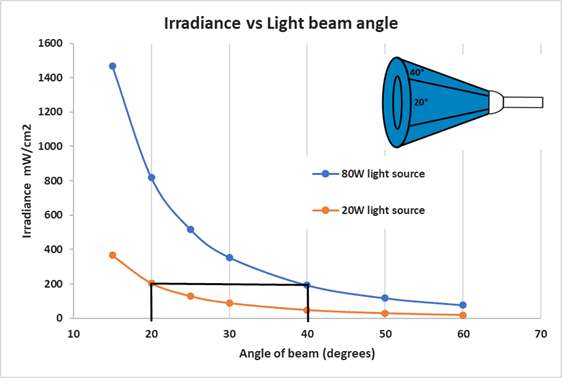
The y-axis represents the light intensity (irradiance) while the x-axis represents the beam’s angle. The chart above demonstrates that a 20 W LED light with 20 degrees of beam angle is as efficient as an 80W LED light with 40 degrees of angle
With irradiance, we are getting closer to the answer, but we are still looking at a 2-D measurement. What we would really like to know is the number of photons being absorbed by the whole reaction (photon flux). Photon flux depends on a number of factors, including the light source (power, spectrum), the position and shape of the reaction vial and the reaction volume. To solve this problem, we need actinominetry. Actinometry is any chemical method for directly measuring the amount of light penetrating your reaction (photon flux). The actinomer is the chemical used to quantify the light. We recently described the actinometric method that we use for determining the light in our photoreactors (Ref 17) using a well established ferrioxalate actinometer (Ref 18).
Ferrioxalate is a versatile actinomer with a range between 250 nm to 500 nm. The Fe(III) compound becomes light sensitive in solution, but stable when kept in the dark. A solution of ferrioxalate can be used in your reaction vial and flask in your reactor as you would set up a standard reaction (albeit in a dark room). Upon irradiation of the sample, the Fe(III) is reduced to Fe(II). Treatment of the Fe(II) species with a phenanthroline solution generates a Fe(II)phenanthroline complex, which can be quantified in comparison to a calibration curve. The amount of Fe(II)phen is proportional to the photons absorbed. Monitoring the time course of irradiation allows you to determine the rate of Fe(II) formation which can be calculated to the photon radian flux in Einsteins/s (energy in one mole of photon). This can be converted for a specific wavelength to determine the number of watts absorbed by the reaction. (a detailed protocol for the synthesis of reagents and description of the math involved can be found in Ref 19).
While it may at first seem complicated, the tools exist to determine directly the photon flux in any reaction setup. With this information, the photon flux from one experiment can be directly compared to any reaction setup as the method is scaled or transferred from lab to lab. Having good familiarity with the above concepts around light, actinometry and photon flux provides a great foundation for the final part of our Getting Started in Photochemistry series where we’ll walk you through setting up your first reactions.
You just read the second part of a three part series designed to help you get started in photochemistry. Below are links to all three parts of the series. Any questions? Send them to info@hepatochem.com, we’d love to hear from you!
Here’s the entire series:
Photochemistry 101, Part I: Everything You Need To Know To Get Started
Photochemistry 101, Part II: Understanding and Measuring Light Sources
Photochemistry 101, Part III: Setting Up Your Initial Photochemistry Reactions
References
- Yes, this is a simplified explanation, there are entire textbooks written about this stuff.
- https://hepatochem.com/red-light-applications-in-photochemistry/
- Don’t worry, there’s still room for you to synthesize 50 nearly identical derivatives of your favorite chromophore.
- https://hepatochem.com/electron-donor-acceptor-eda-complexes-in-photochemistry/
- Tucker, J. and Stephenson, C. R. J. “Shining Light on Photoredox Catalysis: Theory and Synthetic Applications”, Journal of Organic Chemistry, 2012, 77, 1617-1622.
- Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886.
- Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756.
- Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
- Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898−6926. https://pubs.acs.org/doi/abs/10.1021/acs.joc.6b01449
- Romero, N., Nicewicz, “Organic Photoredox Catalysis”, Chemical Reviews, 2016 (116), 10075-10166.
- Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Visible light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem., Int. Ed. 2018, 57, 10034-10072.
- Harper, K. Moschetta, E., Bordawekar, S., Wittenberger, S. “A Laser Driven Flow Chemistry Platform for Scaling Photochemical Reactions with Visible light., ACS Central Science, 2019 (5), 109-115.
- Justin P. Cole, Dian-Feng Chen, Max Kudisch, Ryan M. Pearson, Chern-Hooi Lim, and Garret M. Miyake, “Organocatalyzed Birch Reduction Driven by Visible light, J. Am. Chem. Soc, 2020, 142, 13573-13581. https://pubs.acs.org/doi/abs/10.1021/jacs.0c05899
- Zuo, Z., Ahneman, D., Chu, L., Terrett, J., Doyle, A., Macmillan, D. “Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides” Science, 2014 (345), 437-440.
- Bonfield, H.E., Knauber, T., L©vesque, F. et al. Photons as a 21st century reagent. Nat Commun 11, 804 (2020) https://doi.org/10.1038/s41467-019-13988-4
- https://hepatochem.com/evaluating-light-sources-in-photochemistry/
- https://hepatochem.com/determine-photon-flux-using-actinometry/
- Hatchard C.G.; Parker C.A. “A new sensitive chemical actinometer. 2. Potassium ferrioxalate as a standard chemical actinometer.” Proc. R. Soc. London, Ser. A. 1956, 235, 518-536.
- https://hepatochem.com/standard-ferrioxalate-actinometer-protocol/

